Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takayama, Reona*; Oe, Kazuhiro*; Komori, Yukiko*; Fujisawa, Hiroyuki*; Kuriyama, Ai*; Kikutani, Yuki*; Kikunaga, Hidetoshi*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; et al.
no journal, ,
The extraction behavior of trivalent actinides (Am, Cm, Cf, Es, and Fm) into di(2-ethylhexyl)phosphoric acid, HDEHP, in benzene from HNO was studied together with lanthanides. The extraction constants,
was studied together with lanthanides. The extraction constants, 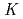
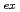 values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of
values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of  Am,
Am,  Cm,
Cm,  Cf, and
Cf, and 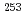 Es, respectively, while that of Fm was measured with
Es, respectively, while that of Fm was measured with 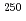 Fm which was produced in the
Fm which was produced in the  U(
U(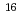 O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The
O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The 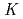
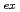 values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the
values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the 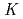
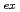 of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.