Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yoshimoto, Masataka*; Tamura, Kazuhisa; Watanabe, Kenta*; Shimizu, Keisuke*; Horisawa, Yuhei*; Kobayashi, Takeshi*; Tsurita, Hanae*; Suzuki, Kota*; Kanno, Ryoji*; Hirayama, Masaaki*
Sustainable Energy & Fuels (Internet), 8(6), p.1236 - 1244, 2024/03
Times Cited Count:0Photo-rechargeable systems, which can efficiently convert and store solar energy into chemical energy within single devices, are essential to harness sunlight effectively. Photo-(de)intercalation plays a pivotal role in the functionality of photorechargeable systems. Nevertheless, the photo-(de)intercalation process has not been conclusively confirmed owing to potential interference from side reactions, such as the decomposition of liquid electrolytes and the elution of electrode materials. In this study, we successfully demonstrated photo-responsive Li -deintercalation using an all-solid-state thin-film battery comprised of epitaxially-grown anatase TiO
-deintercalation using an all-solid-state thin-film battery comprised of epitaxially-grown anatase TiO doped with Nb (a-TiO
doped with Nb (a-TiO :Nb) as the cathode. Under light irradiation, Li
:Nb) as the cathode. Under light irradiation, Li -deintercalation occurred and was subsequently reversibly intercalated into a-TiO
-deintercalation occurred and was subsequently reversibly intercalated into a-TiO :Nb during discharge.
:Nb during discharge.
Watanabe, Kenta*; Horisawa, Yuhei*; Yoshimoto, Masataka*; Tamura, Kazuhisa; Suzuki, Kota*; Kanno, Ryoji*; Hirayama, Masaaki*
Nano Letters, 24(6), p.1916 - 1922, 2024/02
Times Cited Count:1 Percentile:0.02(Chemistry, Multidisciplinary)Electrochemistry has extended from reactions at solid/liquid interfaces to those at solid/solid interfaces. In this study, we achieve the stable photoelectrochemical reaction at the semiconductor-electrode/solid-electrolyte interface in Nb-doped anatase-TiO (a-TiO
(a-TiO :Nb)/Li
:Nb)/Li PO
PO (LPO)/Li all-solid-state cell. The oxidative currents of a-TiO
(LPO)/Li all-solid-state cell. The oxidative currents of a-TiO :Nb/LPO/Li increase upon light irradiation when a-TiO
:Nb/LPO/Li increase upon light irradiation when a-TiO :Nb is located at a potential that is more positive than its flat-band potential. The photoelectrochemical reaction at the semiconductor/solid-electrolyte interface is driven by the same principle as that at semiconductor/liquid-electrolyte interfaces. Thus, we extend photoelectrochemistry to all-solid-state systems composed of solid/solid interfaces.
:Nb is located at a potential that is more positive than its flat-band potential. The photoelectrochemical reaction at the semiconductor/solid-electrolyte interface is driven by the same principle as that at semiconductor/liquid-electrolyte interfaces. Thus, we extend photoelectrochemistry to all-solid-state systems composed of solid/solid interfaces.
Tamura, Kazuhisa
Journal of Physical Chemistry C, 127(46), p.22733 - 22739, 2023/11
Times Cited Count:0 Percentile:0(Chemistry, Physical)The underpotential deposition of Bi on Au(111) electrode in 1 M HClO solution and 1-butyl-3-methylimidazolium tetrafluoroborate was investigated using visible light reflectance measurement and surface X-ray scattering techniques. The electrosorption valency of the UPD reaction of Bi was elucidated and it was found that in both 1 M HClO
solution and 1-butyl-3-methylimidazolium tetrafluoroborate was investigated using visible light reflectance measurement and surface X-ray scattering techniques. The electrosorption valency of the UPD reaction of Bi was elucidated and it was found that in both 1 M HClO and 1-butyl-3-methylimidazolium tetrafluoroborate the electrosorption valency was smaller than 3, but the detail process in the UPD reaction was different between in two electrolytes. The difference may be originated from the difference in the solvation status of Bi
and 1-butyl-3-methylimidazolium tetrafluoroborate the electrosorption valency was smaller than 3, but the detail process in the UPD reaction was different between in two electrolytes. The difference may be originated from the difference in the solvation status of Bi rather than the electrical double layer structure.
rather than the electrical double layer structure.
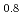 Sr
Sr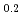 CoO
CoO at initial electrochemical process in an alkaline solution
at initial electrochemical process in an alkaline solutionMatsuzaki, Akira*; Hirayama, Masaaki*; Oguchi, Shoya*; Komo, Mamoru*; Ikezawa, Atsunori*; Suzuki, Kota*; Tamura, Kazuhisa; Arai, Hajime*; Kanno, Ryoji*
Electrochemistry (Internet), 90(10), p.107001_1 - 107001_8, 2022/10
Times Cited Count:0 Percentile:0.01(Electrochemistry)Oxygen reduction and evolution reactions (ORR and OER) of perovskite-type La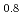 Sr
Sr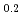 CoO
CoO were characterized using two-dimensional model electrodes with different reaction planes. Synthesized by pulsed laser deposition, these thin and flat electrodes can reveal the reaction plane dependence of the ORR activity. From steady-state polarization measurements in KOH (aq.), the ORR activity was the highest on the (001) film during the first ORR/OER cycle, and it decreased significantly during the second cycle. In-situ synchrotron X-ray diffraction clarified crystal structure changes in the bulk and surface regions of La
were characterized using two-dimensional model electrodes with different reaction planes. Synthesized by pulsed laser deposition, these thin and flat electrodes can reveal the reaction plane dependence of the ORR activity. From steady-state polarization measurements in KOH (aq.), the ORR activity was the highest on the (001) film during the first ORR/OER cycle, and it decreased significantly during the second cycle. In-situ synchrotron X-ray diffraction clarified crystal structure changes in the bulk and surface regions of La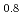 Sr
Sr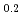 CoO
CoO , and these changes are associated with forming oxygen defects during the initial electrochemical process. Furthermore, the La
, and these changes are associated with forming oxygen defects during the initial electrochemical process. Furthermore, the La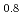 Sr
Sr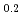 CoO
CoO surface partially decomposed upon reacting. Therefore, the interfacial structures formed in the electrochemical reaction field is important for enhancing ORR and OER activities.
surface partially decomposed upon reacting. Therefore, the interfacial structures formed in the electrochemical reaction field is important for enhancing ORR and OER activities.
Tamura, Kazuhisa; Akutsu-Suyama, Kazuhiro*; Cagnes, M.*; Darwish, T. A.*
ECS Advances (Internet), 1(2), p.020503_1 - 020503_5, 2022/06
The ionic liquid/Si electrode interface was investigated using neutron reflectivity. We precisely elucidated the structure of the electrical double layer formed at 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([BMIM]TFSA)/Si(100) electrode interface with the orientation of the [BMIM]TFSA molecule using a partially deuterated [BMIM]TFSA. The results revealed that [BMIM]TFSA molecules form a layered structure. Cation and anion molecules are alternatingly stacked and molecules in the first three layers are horizontally oriented to the electrode surface at E = -1.2 V, i.e., on the negatively charged electrode surface. It was also revealed that the imidazole ring in [BMIM] cation is parallel to the electrode surface.
Yasuda, Satoshi; Tamura, Kazuhisa; Kato, Masaru*; Asaoka, Hidehito; Yagi, Ichizo*
Journal of Physical Chemistry C, 125(40), p.22154 - 22162, 2021/10
Times Cited Count:10 Percentile:57.69(Chemistry, Physical)Understanding electrochemical behavior of the alkaline metal cation-graphene interface in electrolyte is essential for understanding the fundamental electrochemical interface and development of graphene-based technologies. We report comprehensive analysis of the electrochemical behavior of both alkaline metal cations and graphene using electrochemical surface X-ray diffraction (EC-SXRD) and Raman (EC-Raman) spectroscopic techniques in which the interfacial structure of cations and the charging state and mechanical strain of the graphene can be elucidated. EC-SXRD and cyclic voltammetry demonstrated electrochemically driven specific adsorption and desorption of cations on the graphene surface involved in the dehydration and hydration process. This study provides new insight for understanding fundamental electrochemical behavior of the alkaline metal cation-graphene interface and contributes to the development of carbon-based novel applications.
 MnO
MnO cathode in an all-solid-state thin-film battery during cycling
cathode in an all-solid-state thin-film battery during cyclingHikima, Kazuhiro*; Hinuma, Yoyo*; Shimizu, Keisuke*; Suzuki, Kota*; Taminato, So*; Hirayama, Masaaki*; Masuda, Takuya*; Tamura, Kazuhisa; Kanno, Ryoji*
ACS Applied Materials & Interfaces, 13(6), p.7650 - 7663, 2021/02
Times Cited Count:12 Percentile:67.96(Nanoscience & Nanotechnology)We evaluated the structural change of the cathode material Li MnO
MnO that was deposited as an epitaxial film with an (001) orientation in an all-solid-state battery. In case of the electrode with LiPO
that was deposited as an epitaxial film with an (001) orientation in an all-solid-state battery. In case of the electrode with LiPO coating. Experiments revealed a structural change to a high-capacity (activated) phase that proceeded gradually and continuously with cycling. The activated phase barely showed any capacity fading. We propose a mechanism of structural change with cycling: charging to a high voltage at a sufficiently low Li concentration typically induces irreversible transition to a phase detrimental to cycling that could, but not necessarily, be accompanied by the dissolution of Mn and/or the release of O into the electrolyte, while a gradual irreversible transition to an activated phase happens at a similar Li concentration under a lower voltage.
coating. Experiments revealed a structural change to a high-capacity (activated) phase that proceeded gradually and continuously with cycling. The activated phase barely showed any capacity fading. We propose a mechanism of structural change with cycling: charging to a high voltage at a sufficiently low Li concentration typically induces irreversible transition to a phase detrimental to cycling that could, but not necessarily, be accompanied by the dissolution of Mn and/or the release of O into the electrolyte, while a gradual irreversible transition to an activated phase happens at a similar Li concentration under a lower voltage.
Yasuda, Satoshi; Tamura, Kazuhisa; Terasawa, Tomoo; Yano, Masahiro; Nakajima, Hideaki*; Morimoto, Takahiro*; Okazaki, Toshiya*; Agari, Ryushi*; Takahashi, Yasufumi*; Kato, Masaru*; et al.
Journal of Physical Chemistry C, 124(9), p.5300 - 5307, 2020/03
Times Cited Count:14 Percentile:60.14(Chemistry, Physical)Confinement of hydrogen molecules at graphene-substrate interface has presented significant importance from the viewpoints of development of fundamental understanding of two-dimensional material interface and energy storage system. In this study, we investigate H confinement at a graphene-Au interface by combining selective proton permeability of graphene and the electrochemical hydrogen evolution reaction (electrochemical HER) method. After HER on a graphene/Au electrode in protonic acidic solution, scanning tunneling microscopy finds that H
confinement at a graphene-Au interface by combining selective proton permeability of graphene and the electrochemical hydrogen evolution reaction (electrochemical HER) method. After HER on a graphene/Au electrode in protonic acidic solution, scanning tunneling microscopy finds that H nanobubble structures can be produced between graphene and the Au surface. Strain analysis by Raman spectroscopy also shows that atomic size roughness on the graphene/Au surface originating from the HER-induced strain relaxation of graphene plays significant role in formation of the nucleation site and H
nanobubble structures can be produced between graphene and the Au surface. Strain analysis by Raman spectroscopy also shows that atomic size roughness on the graphene/Au surface originating from the HER-induced strain relaxation of graphene plays significant role in formation of the nucleation site and H storage capacity.
storage capacity.
Yoneda, Yasuhiro; Yoshigoe, Akitaka; Takeda, Yukiharu; Shiwaku, Hideaki; Matsumura, Daiju; Shobu, Takahisa; Tamura, Kazuhisa
Materia, 58(12), p.763 - 769, 2019/12
This is an introduction to the equipment provided for each implementation period belonging to the structure analysis platform in the nanotechnology platform.
Akutsu, Kazuhiro*; Cagnes, M.*; Tamura, Kazuhisa; Kanaya, Toshiji*; Darwish, T. A.*
Physical Chemistry Chemical Physics, 21(32), p.17512 - 17516, 2019/08
Times Cited Count:11 Percentile:53.26(Chemistry, Physical)We combined the deuterium labeling and neutron reflectivity techniques to determine the fine structure of the electric double layer structure in an imidazolium ionic liquid (IL). For this, a simple and large scale deuteration method for imidazolium ILs was developed, where the deuteration level can be systematically controlled.
Kishi, Hirofumi*; Sakamoto, Tomokazu*; Asazawa, Koichiro*; Yamaguchi, Susumu*; Kato, Takeshi*; Zulevi, B.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; Matsumura, Daiju; et al.
Nanomaterials (Internet), 8(12), p.965_1 - 965_13, 2018/12
Times Cited Count:11 Percentile:49.2(Chemistry, Multidisciplinary) PO
PO -modified Li
-modified Li RuO
RuO for lithium-rich layered rocksalt oxide cathodes
for lithium-rich layered rocksalt oxide cathodesTaminato, So*; Hirayama, Masaaki*; Suzuki, Kota*; Kim, K.-S.*; Tamura, Kazuhisa; Kanno, Ryoji*
Journal of Physical Chemistry C, 122(29), p.16607 - 16612, 2018/07
Times Cited Count:8 Percentile:31.15(Chemistry, Physical)Lithium-rich layered rocksalt oxides are promising cathode materials for lithium-ion batteries. We investigate the effects of surface modification by amorphous Li PO
PO on the structures and electrochemical reactions in the surface region of an epitaxial Li
on the structures and electrochemical reactions in the surface region of an epitaxial Li RuO
RuO (010) film electrode. Structural characterization using SXRD, HAXPES, and NR shows that surface modification by Li
(010) film electrode. Structural characterization using SXRD, HAXPES, and NR shows that surface modification by Li PO
PO resulted in the partial substitution of P for Li in the surface region of Li
resulted in the partial substitution of P for Li in the surface region of Li RuO
RuO . The modified (010) surface exhibits better rate capability at 20 C compared to the unmodified surface.
. The modified (010) surface exhibits better rate capability at 20 C compared to the unmodified surface. 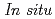 surface XRD confirmed that highly reversible structural changes occurred at the modified surface during lithium (de)intercalation. These results demonstrate that this surface modification stabilizes the crystal structure in the surface region, and it can improve the rate capability of lithium-rich layered rocksalt oxide cathodes.
surface XRD confirmed that highly reversible structural changes occurred at the modified surface during lithium (de)intercalation. These results demonstrate that this surface modification stabilizes the crystal structure in the surface region, and it can improve the rate capability of lithium-rich layered rocksalt oxide cathodes.
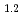 Ni
Ni Mn
Mn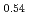 Co
Co O
O in a lithium-ion battery
in a lithium-ion batteryKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 262, p.294 - 300, 2018/06
Times Cited Count:9 Percentile:49.17(Chemistry, Inorganic & Nuclear)The potential in each state of charge (SOC) during charging of Li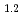 Ni
Ni Mn
Mn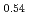 Co
Co O
O is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li
is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li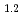 Ni
Ni Mn
Mn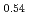 Co
Co O
O was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li
was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li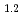 Ni
Ni Mn
Mn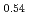 Co
Co O
O in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi
in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi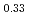 Mn
Mn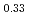 Co
Co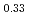 O
O -like and Li
-like and Li MnO
MnO -like, exist in Li
-like, exist in Li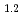 Ni
Ni Mn
Mn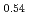 Co
Co O
O .
.
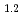 Ni
Ni Mn
Mn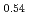 Co
Co O
O used for lithium ion batteries
used for lithium ion batteriesKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 258, p.225 - 231, 2018/02
Times Cited Count:8 Percentile:44.83(Chemistry, Inorganic & Nuclear)Li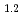 Ni
Ni Mn
Mn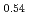 Co
Co O
O is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li
is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li MnO
MnO , LiNi
, LiNi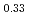 Mn
Mn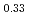 Co
Co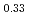 O
O , and Li
, and Li MnO
MnO . On the other hand, in the discharge process, the structure changes in the order of Li
. On the other hand, in the discharge process, the structure changes in the order of Li MnO
MnO and LiNi
and LiNi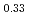 Mn
Mn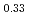 Co
Co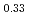 O
O .
.
 and their Zr-O surface modified electrodes
and their Zr-O surface modified electrodesAbe, Machiko*; Iba, Hideki*; Suzuki, Kota*; Minamishima, Hiroaki*; Hirayama, Masaaki*; Tamura, Kazuhisa; Mizuki, Junichiro*; Saito, Tomohiro*; Ikuhara, Yuichi*; Kanno, Ryoji*
Journal of Power Sources, 345, p.108 - 119, 2017/03
Times Cited Count:11 Percentile:38.74(Chemistry, Physical)The surface structure of the Li(Ni, Co, Mn)O electrode was studied during charge/discharge process using electrochemical methods and X-ray/Neutron scattering techniques. It was found that during charge/discharge process the coverage of spinel structure increased. The spinel structure has low electrochemical activity and is not involved in Li insertion/extraction. After the surface modification, it was found that the coverage of the spinel structure did not increase. Further, it was also found out that the Li concentration at the electrode/electrolyte interface increased.
electrode was studied during charge/discharge process using electrochemical methods and X-ray/Neutron scattering techniques. It was found that during charge/discharge process the coverage of spinel structure increased. The spinel structure has low electrochemical activity and is not involved in Li insertion/extraction. After the surface modification, it was found that the coverage of the spinel structure did not increase. Further, it was also found out that the Li concentration at the electrode/electrolyte interface increased.
 O
O /C hydrazine electrooxidation catalysts for anion exchange membrane fuel cells
/C hydrazine electrooxidation catalysts for anion exchange membrane fuel cellsSakamoto, Tomokazu*; Masuda, Teruyuki*; Yoshimoto, Koji*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Matsumura, Daiju; Tamura, Kazuhisa; Hori, Akihiro*; Horiuchi, Yosuke*; Serov, A.*; et al.
Journal of the Electrochemical Society, 164(4), p.F229 - F234, 2017/01
Times Cited Count:13 Percentile:43.47(Electrochemistry)Sakamoto, Tomokazu*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Matsumura, Daiju; Tamura, Kazuhisa; Hori, Akihiro*; Horiuchi, Yosuke*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; et al.
Journal of the Electrochemical Society, 163(10), p.H951 - H957, 2016/08
Times Cited Count:30 Percentile:76.46(Electrochemistry)Tamura, Kazuhisa; Nishihata, Yasuo
Journal of Physical Chemistry C, 120(29), p.15691 - 15697, 2016/07
Times Cited Count:9 Percentile:31.88(Chemistry, Physical)The behavior of halide ions on the Au(111) electrode surface in two ionic liquids (ILs) was investigated by monitoring the structure of the electrode surface. The potential dependences of the X-ray diffraction intensity, which originate from the Au(111)-(1 1) structure and the surface normal structure, were measured simultaneously with cyclic voltammograms. The results revealed that halide ions are co-adsorbed with IL molecules on the electrode surface and increase the mobility of surface atoms. This suggests that the interaction between halide ions and surface Au atoms is weaker than that between IL molecules and surface Au atoms; that is, the surface properties are mainly governed by adsorbed IL molecules. Furthermore, a comparison of the two ILs revealed that the effect of halide ions on the structure of the Au(111) electrode surface depends on the strength of the interaction between IL molecules and surface Au atoms.
1) structure and the surface normal structure, were measured simultaneously with cyclic voltammograms. The results revealed that halide ions are co-adsorbed with IL molecules on the electrode surface and increase the mobility of surface atoms. This suggests that the interaction between halide ions and surface Au atoms is weaker than that between IL molecules and surface Au atoms; that is, the surface properties are mainly governed by adsorbed IL molecules. Furthermore, a comparison of the two ILs revealed that the effect of halide ions on the structure of the Au(111) electrode surface depends on the strength of the interaction between IL molecules and surface Au atoms.
 surface under high voltage battery operation
surface under high voltage battery operationTaminato, So*; Hirayama, Masaaki*; Suzuki, Kota*; Tamura, Kazuhisa; Minato, Taketoshi*; Arai, Hajime*; Uchimoto, Yoshiharu*; Ogumi, Zempachi*; Kanno, Ryoji*
Journal of Power Sources, 307, p.599 - 603, 2016/03
Times Cited Count:34 Percentile:71.85(Chemistry, Physical)An epitaxial-film model electrode of LiCoO (104) was fabricated on SrRuO
(104) was fabricated on SrRuO (100)/Nb:SrTiO
(100)/Nb:SrTiO (100) using pulsed laser deposition. The 50 nm thick LiCoO
(100) using pulsed laser deposition. The 50 nm thick LiCoO (104) film exhibited lithium (de-)intercalation activity with a first discharge capacity of 119 mAh g
(104) film exhibited lithium (de-)intercalation activity with a first discharge capacity of 119 mAh g between 3.0 and 4.4 V, followed by a gradual capacity fading with subsequent charge-discharge cycles. In contrast, a 3.2 nm thick Li
between 3.0 and 4.4 V, followed by a gradual capacity fading with subsequent charge-discharge cycles. In contrast, a 3.2 nm thick Li PO
PO -coated film exhibited a higher intercalation capacity of 148 mAh g
-coated film exhibited a higher intercalation capacity of 148 mAh g with superior cycle retention than the uncoated film. In situ surface X-ray diffraction measurements revealed a small lattice change at the coated surface during the (de-)intercalation processes compared to the uncoated surface. The surface modification of LiCoO
with superior cycle retention than the uncoated film. In situ surface X-ray diffraction measurements revealed a small lattice change at the coated surface during the (de-)intercalation processes compared to the uncoated surface. The surface modification of LiCoO by the Li
by the Li PO
PO coating could lead to improvement of the structural stability at the surface region during lithium (de-)intercalation at high voltage.
coating could lead to improvement of the structural stability at the surface region during lithium (de-)intercalation at high voltage.
Sakamoto, Tomokazu*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo
Hyomen Kagaku, 37(2), p.78 - 83, 2016/02
We have developed direct liquid fuel anion exchange membrane fuel cell vehicles to deal with the global warming. Non-platinum group metals (PGM) catalyst has been researched to apply for both anode and cathode electrodes. A test driving was carried out for the fuel cell vehicle equipped with no precious metals as catalysts at SPring-8 in 2013. Here we introduce our results of advanced analysis for reaction mechanism and active site of non-PGM catalyst using synchrotron radiation X-rays at SPring-8.