Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 nanosheets for enhancing heat storage performance
nanosheets for enhancing heat storage performanceYoshisako, Hiroki*; Okamoto, Norihiko*; Tanaka, Kazuya; Ichitsubo, Tetsu
Communications Chemistry (Internet), 8, p.169_1 - 169_9, 2025/06
no abstracts in English
Kawasaki, Takuro; Fukuda, Tatsuo; Yamanaka, Satoru*; Murayama, Ichiro*; Kato, Takanori*; Baba, Masaaki*; Hashimoto, Hideki*; Harjo, S.; Aizawa, Kazuya; Tanaka, Hirohisa*; et al.
Journal of Applied Physics, 137(9), p.094101_1 - 094101_7, 2025/03
Times Cited Count:0 Percentile:0.00(Physics, Applied)Vu, TheDang*; Shishido, Hiroaki*; Aizawa, Kazuya; Oku, Takayuki; Oikawa, Kenichi; Harada, Masahide; Kojima, Kenji M*; Miyajima, Shigeyuki*; Soyama, Kazuhiko; Koyama, Tomio*; et al.
IEEJ Transactions on Electrical and Electronic Engineering, 19(11), p.1888 - 1894, 2024/11
Times Cited Count:0 Percentile:0.00(Engineering, Electrical & Electronic)Ohgama, Kazuya; Doda, Norihiro; Uwaba, Tomoyuki; Futagami, Satoshi; Tanaka, Masaaki; Yamano, Hidemasa; Ota, Hirokazu*; Ogata, Takanari*; Wozniak, N.*; Shemon, E.*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle (GLOBAL2024) (Internet), 4 Pages, 2024/10
To enhance the accuracy of the safety evaluations in sodium-cooled fast reactors, it is necessary to develop a method to realistically evaluate the reactivity caused by core deformation. In this regard, Japan and the United States jointly conducted a benchmark analysis of thermal bowing experiments of a single duct of Joyo-type fuel assembly. The aim was to confirm the validity of the core bowing analysis codes. Comparisons of analysis and test results revealed that the core bowing analysis codes used by both countries were able to reasonably predict the axial distribution of horizontal duct displacement of a single duct due to thermal bowing and the contact load on the duct pad.
Wozniak, N.*; Shemon, E.*; Feng, B.*; Ohgama, Kazuya; Doda, Norihiro; Uwaba, Tomoyuki; Futagami, Satoshi; Tanaka, Masaaki; Yamano, Hidemasa; Ota, Hirokazu*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle (GLOBAL2024) (Internet), 4 Pages, 2024/10
To enhance the accuracy of the safety evaluations in sodium-cooled fast reactors, it is necessary to develop a method to realistically evaluate the reactivity caused by core deformation. In this regard, Japan and the United States jointly conducted a benchmark analysis of thermal bowing experiments using multiple ducts of Joyo-type fuel assembly. The aim was to confirm the validity of the core bowing analysis codes. Comparisons of analysis and test results revealed that the core bowing analysis codes used by both countries were able to reasonably predict the thermal bowing of a row of assemblies.
Tian, Q.*; Feng, L.*; Wu, C.*; Wen, J.*; Qiu, X.*; Tanaka, Kazuya; Onuki, Toshihiko*; Yu, Q.*
Journal of Colloid and Interface Science, 669, p.1006 - 1014, 2024/09
Times Cited Count:2 Percentile:31.33(Chemistry, Physical)Vu, TheDang*; Shishido, Hiroaki*; Aizawa, Kazuya; Oku, Takayuki; Oikawa, Kenichi; Harada, Masahide; Kojima, Kenji M*; Miyajima, Shigeyuki*; Soyama, Kazuhiko; Koyama, Tomio*; et al.
Journal of Physics; Conference Series, 2776, p.012009_1 - 012009_9, 2024/06
Mikami, Satoshi; Tokiyoshi, Masanori*; Sato, Rina*; Tanaka, Daisuke*; Yoshimura, Kazuya
Nihon Hoshasen Anzen Kanri Gakkai-Shi, 23(1), p.10 - 17, 2024/06
Taisei Corporation and Infocube LAFLA Co., Ltd. have developed the smartphone-wirelessly-connected dosemeters, aiming to apply it to real-time exposure management of multiple decontamination workers. In order to grasp the basic characteristics of the developed dosemeters, they were calibrated and tested their characteristics such as energy dependency, angle of incidence dependency, etc. at photon calibration fields in Japan Atomic Energy Agency. The results showed generally good characteristics in each test. We evaluated that the dosemeters can be effectively used for decontamination work.
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the  Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10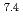 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:6 Percentile:72.77(Chemistry, Physical)no abstracts in English
Mei, H.; Aoyagi, Noboru; Saito, Takumi*; Tanaka, Kazuya; Sugiura, Yuki; Tachi, Yukio
Applied Geochemistry, 162, p.105926_1 - 105926_8, 2024/02
Times Cited Count:2 Percentile:69.68(Geochemistry & Geophysics)Ishida, Takekazu*; Vu, TheDang*; Shishido, Hiroaki*; Aizawa, Kazuya; Oku, Takayuki; Oikawa, Kenichi; Harada, Masahide; Kojima, Kenji M*; Miyajima, Shigeyuki*; Koyama, Tomio*; et al.
Journal of Low Temperature Physics, 214(3-4), p.152 - 157, 2024/02
Times Cited Count:0 Percentile:0.00(Physics, Applied)Hamase, Erina; Ohgama, Kazuya; Kawamura, Takumi*; Doda, Norihiro; Tanaka, Masaaki; Yamano, Hidemasa
Annals of Nuclear Energy, 195, p.110157_1 - 110157_14, 2024/01
Times Cited Count:3 Percentile:62.75(Nuclear Science & Technology)To validate the fast reactor plant dynamics analysis code Super-COPD for the loss of flow without scram (LOFWOS) event, we participated in the IAEA benchmark for the LOFWOS test No.13 performed at the FFTF as one of the passive safety demonstration test. In the blind phase, there were challenges to reproduce outlet temperatures of fuel assemblies and the total reactivity. To improve the evaluation accuracy of them, the whole core model considering the radial heat transfer and interwrapper flow and the simplified assembly bowing reactivity model were introduced. As a result of the final phase, the second peak of outlet temperatures was reproduced successfully, and the total reactivity could generally follow the measured data. Super-COPD was validated for the LOFWOS event.
Terasawa, Tomoo; Matsunaga, Kazuya*; Hayashi, Naoki*; Ito, Takahiro*; Tanaka, Shinichiro*; Yasuda, Satoshi; Asaoka, Hidehito
Vacuum and Surface Science, 66(9), p.525 - 530, 2023/09
As Au (001) surfaces exhibit a quasi-one-dimensional corrugated structure, Hex-Au(001), its periodicity was predicted to change the electronic structure of graphene when graphene was grown on this surface. Furthermore, the hybridization between graphene and Au is known to introduce bandgap and spin polarization into graphene. Here, we report angle-resolved photoemission spectroscopy and density functional theory calculation of graphene on a Hex-Au(001) surface. A bandgap of 0.2 eV in the graphene Dirac cone was observed at the crossing point of the graphene Dirac cone and Au 6sp bands, indicating that the origin of the bandgap formation was the hybridization between the graphene Dirac cone and Au 6sp band. We discussed the hybridization mechanism and anticipated spin injection into the graphene Dirac cone.
 single crystals based on Bragg-dip analysis using a delay-line superconducting sensor
single crystals based on Bragg-dip analysis using a delay-line superconducting sensorShishido, Hiroaki*; Vu, TheDang*; Aizawa, Kazuya; Kojima, Kenji M*; Koyama, Tomio*; Oikawa, Kenichi; Harada, Masahide; Oku, Takayuki; Soyama, Kazuhiko; Miyajima, Shigeyuki*; et al.
Journal of Applied Crystallography, 56(4), p.1108 - 1113, 2023/08
Times Cited Count:2 Percentile:43.78(Chemistry, Multidisciplinary)Doda, Norihiro; Kato, Shinya; Hamase, Erina; Kuwagaki, Kazuki; Kikuchi, Norihiro; Ohgama, Kazuya; Yoshimura, Kazuo; Yoshikawa, Ryuji; Yokoyama, Kenji; Uwaba, Tomoyuki; et al.
Proceedings of 20th International Topical Meeting on Nuclear Reactor Thermal Hydraulics (NURETH-20) (Internet), p.946 - 959, 2023/08
An innovative design system named ARKADIA is being developed to realize the design of advanced nuclear reactors as safe, economical, and sustainable carbon-free energy sources. This paper focuses on ARKADIA-Design for design studies as a part of ARKADIA and introduces representative verification methods for numerical analysis methods of the core design. ARKADIA-Design performs core performance analysis of sodium-cooled fast reactors using a multiphysics approach that combines neutronics, thermal-hydraulics, core mechanics, and fuel pin behavior analysis codes. To confirm the validity of these analysis codes, validation matrices are identified with reference to experimental data and reliable numerical analysis results. The analysis models in these codes and the representative practices for the validation matrices are described.
Maamoun, I.; Falyouna, O.*; Shariful, I. M.*; Eljamal, R.*; Bensaida, K.*; Tanaka, Kazuya; Tokunaga, Kohei; Eljamal, O.*
Advanced Materials Letters (Internet), 14(2), p.23021721_1 - 23021721_10, 2023/04
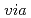 bimetallic Ni/Fe
bimetallic Ni/Fe nanoparticles; Implications towards Tc(VII) removal
nanoparticles; Implications towards Tc(VII) removalMaamoun, I.; Tokunaga, Kohei; Dohi, Terumi; Kanno, Futoshi*; Falyouna, O.*; Eljamal, O.*; Tanaka, Kazuya
Frontiers in Nuclear Engineering (Internet), 2, p.1142823_1 - 1142823_17, 2023/03
Recently, the rapid development of nuclear power technologies and the continuous energy demand around the world exhibited massive amounts of contaminated water with radionuclides. Technetium-99 ( Tc) is one of the long-lived radionuclides with a highly mobile anionic form (Tc(VII)) in aqueous solutions. Hence, the rapid and efficient Tc(VII) removal from radioactive water has emerged as a challenging issue. In this work, bimetallic nickel/iron nanoparticles (Ni/Fe
Tc) is one of the long-lived radionuclides with a highly mobile anionic form (Tc(VII)) in aqueous solutions. Hence, the rapid and efficient Tc(VII) removal from radioactive water has emerged as a challenging issue. In this work, bimetallic nickel/iron nanoparticles (Ni/Fe ) were prepared to enhance the immobilization of rhenium (Re(VII)) from aqueous solutions, as the surrogate of Tc(VII).
) were prepared to enhance the immobilization of rhenium (Re(VII)) from aqueous solutions, as the surrogate of Tc(VII).
Doda, Norihiro; Uwaba, Tomoyuki; Ohgama, Kazuya; Yoshimura, Kazuo; Nemoto, Toshiyuki*; Tanaka, Masaaki; Yamano, Hidemasa
Nihon Kikai Gakkai Kanto Shibu Dai-29-Ki Sokai, Koenkai Koen Rombunshu (Internet), 5 Pages, 2023/03
An evaluation method for reactivity feedback due to core deformation during reactor power increase in sodium-cooled fast reactors is being developed for realistic core design evaluation. In this evaluation method, fuel assembly bowing was modeled with a beam element of the finite element method, and the assembly's pad contact between adjacent assemblies was modeled with a dedicated element which could consider the wrapper tube cross-sectional distortion and the pad stiffness depending on pad contact conditions. This fuel assembly bowing analysis model was verified for thermal bowing of a single assembly and assembly pad contact between adjacent assemblies in a core as past benchmark problems. The calculation results by this model showed good agreement with those of reference solutions of theoretical solutions or results by participating institutions in the benchmark. This study confirmed that the analysis model was able to calculate thermal assembly bowing appropriately.

 -, SeO
-, SeO
 -, and SeO
-, and SeO
 -coprecipitated barite after treatment with phosphate
-coprecipitated barite after treatment with phosphateTokunaga, Kohei; Tanaka, Kazuya; Takahashi, Yoshio*; Kozai, Naofumi
Environmental Science & Technology, 57(8), p.3166 - 3175, 2023/02
Times Cited Count:4 Percentile:44.52(Engineering, Environmental)Coprecipitation of radionuclides with barite has been studied to remove radionuclides from radioactive liquid waste because of its excellent removal efficiency; however, little information exists concerning the stability of the ions coprecipitated with barite. This study systematically investigated the stability of iodate, selenite, and selenate coprecipitated with barite via leaching tests. These oxyanions were gradually leached from the oxyanion-bearing barite into ultrapure water over time. Leaching of the oxyanions significantly increased in leaching solutions containing NaCl (pH5.3), NaNO (pH5.9), and Na
(pH5.9), and Na SO
SO (pH5.7). Conversely, leaching of the oxyanions was suppressed in KH
(pH5.7). Conversely, leaching of the oxyanions was suppressed in KH PO
PO solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO
solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.
was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.