Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:3 Percentile:34.10(Chemistry, Multidisciplinary)no abstracts in English
Narita, Hirokazu*; Kasuya, Ryo*; Suzuki, Tomoya*; Motokawa, Ryuhei; Tanaka, Mikiya*
Encyclopedia of Inorganic and Bioinorganic Chemistry (Internet), 28 Pages, 2020/12
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:5 Percentile:17.86(Chemistry, Analytical)no abstracts in English
Narita, Hirokazu*; Nicolson, R. M.*; Motokawa, Ryuhei; Ito, Fumiyuki*; Morisaku, Kazuko*; Goto, Midori*; Tanaka, Mikiya*; Heller, W. T.*; Shiwaku, Hideaki; Yaita, Tsuyoshi; et al.
Inorganic Chemistry, 58(13), p.8720 - 8734, 2019/07
Times Cited Count:20 Percentile:75.87(Chemistry, Inorganic & Nuclear)Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Metals, 8(7), p.558_1 - 558_10, 2018/07
Times Cited Count:17 Percentile:59.49(Materials Science, Multidisciplinary)The refining of platinum group metals is based mainly on solvent extraction methods, whereas Ru is selectively recovered by distillation as RuO . Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl
. Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl (H
(H O)
O) ]
] to [RuCl
to [RuCl ]
]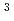
 with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru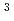
 had 5 Cl
had 5 Cl and 1 H
and 1 H O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl
O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl (H
(H O)]
O)]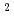
 on [RuCl
on [RuCl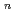 (H
(H O)
O)
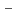
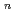 ]
]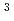

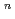 suggested that the EHBAA extracted the pentachlorido species.
suggested that the EHBAA extracted the pentachlorido species.
Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Motokawa, Ryuhei; Shiwaku, Hideaki; Yaita, Tsuyoshi
Analytical Sciences, 33(11), p.1305 - 1309, 2017/11
Times Cited Count:11 Percentile:36.78(Chemistry, Analytical)Maeda, Motoki*; Narita, Hirokazu*; Tokoro, Chiharu*; Tanaka, Mikiya*; Motokawa, Ryuhei; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation and Purification Technology, 177, p.176 - 181, 2017/04
Times Cited Count:22 Percentile:55.45(Engineering, Chemical)Kamiji, Yu; Taniguchi, Masashi*; Nishihata, Yasuo; Nagaishi, Ryuji; Tanaka, Hirohisa*; Hirata, Shingo*; Hara, Mikiya; Hino, Ryutaro
E-Journal of Advanced Maintenance (Internet), 7(1), p.84 - 89, 2015/05
For hydrogen mitigation, a new type passive autocatalytic recombiner is under developing. This new recombiner has been developed from automotive monolithic catalyst in order to reduce weight and to improve hydrogen treating capacity, environmental resistance and product quality. In this study, activation energy of hydrogen-oxygen recombination reaction was examined to clarify the basic characteristics of the catalyst. In addition, the degradation of the catalyst by  -ray irradiation simulating the environmental condition in nuclear power plants was also examined. As a result, the activation energy was experimentally estimated at 5.75 kJ/mol. Besides, no significant differences were observed in compositional distribution from the EPMA results. On the other hand, specific surface area of the catalyst and surface area of the precious metals were increased. Moreover, catalyst performance test showed that
-ray irradiation simulating the environmental condition in nuclear power plants was also examined. As a result, the activation energy was experimentally estimated at 5.75 kJ/mol. Besides, no significant differences were observed in compositional distribution from the EPMA results. On the other hand, specific surface area of the catalyst and surface area of the precious metals were increased. Moreover, catalyst performance test showed that  -ray irradiation up to 1.0 MGy can increase activity of catalyst.
-ray irradiation up to 1.0 MGy can increase activity of catalyst.
Kamiji, Yu; Matsumura, Daiju; Taniguchi, Masashi*; Nishihata, Yasuo; Tanaka, Hirohisa*; Hirata, Shingo*; Hara, Mikiya; Hino, Ryutaro
Proceedings of 23rd International Conference on Nuclear Engineering (ICONE-23) (DVD-ROM), 4 Pages, 2015/05
In a severe accident at a nuclear power plant, a large amount of hydrogen can be released to primary containment vessel or reactor building. Passive autocatalytic recombiner (PAR) is one of the most effective systems for hydrogen mitigation and safety accident management. The new type PAR is under developing to improve conventional PARs, especially its size and weight. In this study, the influence of steam coexistence for the automotive catalyst activity was experimentally examined. These results show that the steam slightly affects the reaction start up and catalyst activity.
Kamiji, Yu; Taniguchi, Masashi*; Nishihata, Yasuo; Nagaishi, Ryuji; Tanaka, Hirohisa*; Hirata, Shingo*; Hara, Mikiya; Hino, Ryutaro
Proceedings of 2nd International Conference on Maintenance Science and Technology (ICMST-Kobe 2014), p.87 - 88, 2014/11
For hydrogen mitigation, a new type passive autocatalytic recombiner is under development. In this study, the activation energy of hydrogen-oxygen recombination reaction was examined to clarify the basic characteristics of the catalyst. In addition, the degradation of the catalyst by  -ray irradiation simulating the environmental condition in nuclear power plants was also examined. As a result, the activation energy was experimentally estimated at 5.75 kJ/mol. Besides, no significant differences were observed in the compositional distribution from the EPMA results between the non-irradiated and the irradiated catalyst. However, the irradiated catalyst showed much more activity because of larger specific surface area of the catalyst and surface area of the precious metals. It showed that
-ray irradiation simulating the environmental condition in nuclear power plants was also examined. As a result, the activation energy was experimentally estimated at 5.75 kJ/mol. Besides, no significant differences were observed in the compositional distribution from the EPMA results between the non-irradiated and the irradiated catalyst. However, the irradiated catalyst showed much more activity because of larger specific surface area of the catalyst and surface area of the precious metals. It showed that  -ray irradiation up to 1.0 MGy can increase activity of the catalyst.
-ray irradiation up to 1.0 MGy can increase activity of the catalyst.
Narita, Hirokazu*; Tanaka, Mikiya*; Sato, Yumiko*; Yaita, Tsuyoshi; Okamoto, Yoshihiro
Solvent Extraction and Ion Exchange, 24(5), p.693 - 702, 2006/05
Times Cited Count:14 Percentile:46.70(Chemistry, Multidisciplinary)The structure of the Ni(II) complex extracted with the commercial hydroxyoxime, LIX84I, and the effect of adding bis(2-ethylhexyl)phosphoricacid(D2EHPA) to LIX84I on the extraction rate and the coordination of Ni(II) were investigated by solvent extraction and XAFS methods. The XANES spectrum and the curve fit soft the EXAFS spectrum of the Ni-LIX84I complex showed that the complex is four-coordinate square-planar with a 1:2 stoichiometry. In the Ni(II)-D2EHPA-LIX84I system, the coordination geometry changes to six-coordinate octahedral with an increase in the D2EHPA concentration. Although the rate of Ni(II) extraction with LIX84I is significantly accelerated by adding as small amount of D2EHPA, most of the Ni(II) complexes extracted with this organic solution remain square-planar. This indicates that the effect of D2EHPA on the increase in the extraction rate would be attributed to a behavior of this ligand like catalysis.
Motokawa, Ryuhei; Suzuki, Shinichi; Yaita, Tsuyoshi; Narita, Hirokazu*; Tanaka, Mikiya*
no journal, ,
no abstracts in English
Motokawa, Ryuhei; Narita, Hirokazu*; Tanaka, Mikiya*; Suzuki, Shinichi; Yaita, Tsuyoshi
no journal, ,
no abstracts in English
Naganawa, Hirochika; Tanaka, Mikiya*; Saki, Yukinori*
no journal, ,
Electroless nickel plating is an important surface finishing technology; however, the treatment of a large amount of spent baths should be established from both the environmental and economic viewpoints. Solvent extraction is a useful separation technology which utilizes the distribution of a solute between aqueous and organic phases. The present authors have developed a nickel recycling process from the spent baths using solvent extraction. This process was applied to a plating plant initially using a conventional mixer-settler and then a new apparatus called emulsion flow extractor. In this presentation, the laboratory-scale experimental results and practical operation data will be presented.
Motokawa, Ryuhei; Narita, Hirokazu*; Suzuki, Shinichi; Tanaka, Mikiya*; Yaita, Tsuyoshi
no journal, ,
no abstracts in English
Motokawa, Ryuhei; Suzuki, Shinichi; Yaita, Tsuyoshi; Narita, Hirokazu*; Tanaka, Mikiya*
no journal, ,
no abstracts in English