Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tokuhisa, Atsushi*; Arai, Junya*; Jochi, Yasumasa*; Ono, Yoshiyuki*; Kameyama, Toyohisa*; Yamamoto, Keiji*; Hatanaka, Masayuki*; Gerofi, B.*; Shimada, Akio*; Kurokawa, Motoyoshi*; et al.
Journal of Synchrotron Radiation, 20(6), p.899 - 904, 2013/11
Times Cited Count:5 Percentile:29.23(Instruments & Instrumentation)Yabashi, Makina*; Tanaka, Hitoshi*; Tanaka, Takashi*; Tomizawa, Hiromitsu*; Togashi, Tadashi*; Nagasono, Mitsuru*; Ishikawa, Tetsuya*; Harries, J.; Hikosaka, Yasumasa*; Hishikawa, Akiyoshi*; et al.
Journal of Physics B; Atomic, Molecular and Optical Physics, 46(16), p.164001_1 - 164001_19, 2013/08
Times Cited Count:71 Percentile:95.16(Optics) -methyltransferase involved in flower coloration
-methyltransferase involved in flower colorationAkita, Yusuke; Kitamura, Satoshi; Hase, Yoshihiro; Narumi, Issei; Ishizaka, Hiroshi*; Kondo, Emiko*; Kameari, Naoko*; Nakayama, Masayoshi*; Tanikawa, Natsu*; Morita, Yasumasa*; et al.
Planta, 234(6), p.1127 - 1136, 2011/12
Times Cited Count:41 Percentile:75.95(Plant Sciences) spp., generated by ion-beam irraidation
spp., generated by ion-beam irraidationKondo, Emiko*; Nakayama, Masayoshi*; Kameari, Naoko*; Tanikawa, Natsu*; Morita, Yasumasa*; Akita, Yusuke; Hase, Yoshihiro; Tanaka, Atsushi; Ishizaka, Hiroshi*
JAEA-Review 2010-065, JAEA Takasaki Annual Report 2009, P. 65, 2011/01
no abstracts in English
 spp., generated by ion-beam irradiation
spp., generated by ion-beam irradiationKondo, Emiko*; Nakayama, Masayoshi*; Kameari, Naoko*; Tanikawa, Natsu*; Morita, Yasumasa*; Akita, Yusuke; Hase, Yoshihiro; Tanaka, Atsushi; Ishizaka, Hiroshi*
Plant Biotechnology, 26(5), p.565 - 569, 2009/01
Times Cited Count:33 Percentile:66.84(Biotechnology & Applied Microbiology)Fragrant cyclamen cultivar (

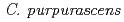 ), that bloomed purple flower containing malvidin 3,5-diglucoside as the major anthocyanin, was irradiated with a 320-MeV carbon-ion beam at 0-16 Gy to increase flower color variation by mutation. Some of the M2 plants derived from self-pollination of M1 plants irradiated at 2 Gy were flower-color mutants that retained desirable flower shape, flower size, and leaf color. One of the mutants bloomed novel red-purple flowers, the major anthocyanin of which was delphinidin 3,5-diglucoside. Because the major anthocyanins in flowers of
), that bloomed purple flower containing malvidin 3,5-diglucoside as the major anthocyanin, was irradiated with a 320-MeV carbon-ion beam at 0-16 Gy to increase flower color variation by mutation. Some of the M2 plants derived from self-pollination of M1 plants irradiated at 2 Gy were flower-color mutants that retained desirable flower shape, flower size, and leaf color. One of the mutants bloomed novel red-purple flowers, the major anthocyanin of which was delphinidin 3,5-diglucoside. Because the major anthocyanins in flowers of  spp. were previously restricted to malvidin, peonidin, and cyanidin types, the generation of a cyclamen containing mostly the delphinidin-type anthocyanin is an important breakthrough in cyclamen breeding. We expect this mutant to become not only a commercial cultivar itself, but also a valuable genetic resource for cyclamen breeding.
spp. were previously restricted to malvidin, peonidin, and cyanidin types, the generation of a cyclamen containing mostly the delphinidin-type anthocyanin is an important breakthrough in cyclamen breeding. We expect this mutant to become not only a commercial cultivar itself, but also a valuable genetic resource for cyclamen breeding.
Sano, Yuichi; Karino, Junichi*; Koyama, Tomozo; Tanaka, Yasumasa
PNC TN8410 98-053, 67 Pages, 1998/03
The coordination properties of extracted HNO and lanthanide (Ln) complexes in TRUEX process were studied by NMR (nuclear magnetic resonance) measurements. The main results are shown as follows; (1)In the HNO
and lanthanide (Ln) complexes in TRUEX process were studied by NMR (nuclear magnetic resonance) measurements. The main results are shown as follows; (1)In the HNO extraction with TRUEX solvent, coordination manner of CMPO to HNO
extraction with TRUEX solvent, coordination manner of CMPO to HNO changes with the HNO
changes with the HNO concentration in the aqueous phase. (2)The mole ratio of coordinated ligands (TBP and CMPO) to free ligands in the heavy organic phase (third phase), which was generated from the TRUEX solvents after extracting HNO
concentration in the aqueous phase. (2)The mole ratio of coordinated ligands (TBP and CMPO) to free ligands in the heavy organic phase (third phase), which was generated from the TRUEX solvents after extracting HNO , is larger than that in the light organic phase. (3)In the Ln extraction with TRUEX solvents, only CMPO coordinates to the lanthanide ion, and TBP doesn't exist within the first coordination sphere. (4)The eontribution of CMPO carbonyl to the Ln extraction will become larger with the extraction of HNO
, is larger than that in the light organic phase. (3)In the Ln extraction with TRUEX solvents, only CMPO coordinates to the lanthanide ion, and TBP doesn't exist within the first coordination sphere. (4)The eontribution of CMPO carbonyl to the Ln extraction will become larger with the extraction of HNO . (5)The CMPO coordinates to Ln in the bidentate manner, which is independent of Ln elements and the phase separation. (6)About the CMPO exchange reaction in the extracted Ln complexes, the following results were obtained ; (a)The activation parameters (exchange rate constants, activation enthalpy and activation entropy) decrease with the increase of atomic number of extracted Ln, which may be caused by the interaction with TBP in the outer sphere. (b)The exchange rate constants in the heavy organic phase are larger than that in the light organic phase.
. (5)The CMPO coordinates to Ln in the bidentate manner, which is independent of Ln elements and the phase separation. (6)About the CMPO exchange reaction in the extracted Ln complexes, the following results were obtained ; (a)The activation parameters (exchange rate constants, activation enthalpy and activation entropy) decrease with the increase of atomic number of extracted Ln, which may be caused by the interaction with TBP in the outer sphere. (b)The exchange rate constants in the heavy organic phase are larger than that in the light organic phase.
 -ray irradiation effect on corrosion rates of stsinless steel in boilingnitric acid containi
-ray irradiation effect on corrosion rates of stsinless steel in boilingnitric acid containi; Nagai, Takayuki; Takeda, Seiichiro; Tanaka, Yasumasa
Journal of Nuclear Science and Technology, 35(5), p.353 - 356, 1998/00
None
; Nagai, Takayuki; Takeda, Seiichiro; Tanaka, Yasumasa
Journal of Nuclear Science and Technology, 35(5), p.353 - 356, 1998/00
Times Cited Count:12 Percentile:66.43(Nuclear Science & Technology)None
Koma, Yoshikazu; Shibata, Atsuhiro; Koyama, Tomozo; Tanaka, Yasumasa; Yamana, Hajimu*
PNC TN8410 97-068, 46 Pages, 1997/03
None
Ozawa, Masaki; Tanaka, Yasumasa; Ohara, Chisako*; Tanuma, Hiroyuki*
Proceedings of International Conference on Future Nuclear Systems (GLOBAL'97), p.1232 - 1237, 1997/00
None
Ozawa, Masaki; Tanaka, Yasumasa; Baron, P.*
Proceedings of International Conference on Future Nuclear Systems (GLOBAL'97), 0 Pages, 1997/00
None
Sano, Yuichi; *; ; *; Koyama, Tomozo; Tanaka, Yasumasa
PNC TN8410 96-362, 19 Pages, 1996/10
The coordination properties of the lanthanide (La, Ce, Pr, Nd, Sm and Eu) complexes in lanthanide/TBP (tributylphosphate), lanthanide/CMPO (octyl(phenyl)-N,N-diisobutycarbamoylmethylphosphine oxide) and lanthanide/CMPO/TBP systems were investigated by NMR (nuclear magnetic resonance) measurements. In the lanthanide/CMPO/TBP system, it is shown that the structure of lanthanide complex is changed by the concentration ratio for added CMPO to the lanthanide ion ; lanthanide/CMPO/TBP system ([CMPO]/[Ln]  3 (mole ratio)) NO
3 (mole ratio)) NO - the similar coordination in the Ln/TBP system TBP, CMPO - These coordinate to the lanthanide ion together. (the existence of several complexes) lanthanide/CMPO/TBP system ([CMPO]/[Ln}
- the similar coordination in the Ln/TBP system TBP, CMPO - These coordinate to the lanthanide ion together. (the existence of several complexes) lanthanide/CMPO/TBP system ([CMPO]/[Ln}  3(mole ratio)) NO
3(mole ratio)) NO - the same coordination in the Ln/CMPO system TBP, CMPO - Only CMPO coordinates to the lanthanide ion. TBP doesn't exist within the first coordination sphere, but affects the CMPO exchange reaction.
- the same coordination in the Ln/CMPO system TBP, CMPO - Only CMPO coordinates to the lanthanide ion. TBP doesn't exist within the first coordination sphere, but affects the CMPO exchange reaction.
Koma, Yoshikazu; Watanabe, Masayuki; Nemoto, Shinichi; Ozawa, Masaki; Okamoto, Fumitoshi; Tanaka, Yasumasa
PNC TN8410 95-193, 49 Pages, 1995/06
None
Shibata, Atsuhiro; P.Y.COR*; *; ; ; *; Tanaka, Yasumasa
PNC TN8410 95-112, 100 Pages, 1995/05
PNC has developed the TRUEX process to recover transuranium elements from high level liquid waste. This process uses CMPO as extracting solvent. DIAMEX process which uses Diamide has been developed bY CEA in France. The aim of basic studies was to get some comparison data between CMPO and Diamide. We have conducted the experiments regarding properties of Ln(III) extraction and behavior of third phase formation. Also, we have investigated properties of Ln(III) extraction with TBP and DBBP to compare bidentate ligand with monodentate ligand. This study was conducted as a frame of PNC/CEA collaboration on minor Actinide partitioning. The conclusions drawn from our studies can be summarized as follows; (1)Trivalent Lanthanides extraction reaction. CMPO, TBP and DBBP ; Ln + 3NO
+ 3NO + 3Extractant
+ 3Extractant  Ln(NO
Ln(NO )
)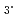 3Extractant. Diamide; Ln
3Extractant. Diamide; Ln + 3NO
+ 3NO + m Diamide
+ m Diamide 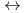 Ln(NO
Ln(NO )
)
 m Diamide : m=O.7
m Diamide : m=O.7 1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO
1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO  Diamide
Diamide  DBBP
DBBP  TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2
TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2  10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
Ishibashi, Yuzo; Kawata, Tomio; Tanaka, Yasumasa; ;
Proceedings of 3rd International Conference on Nuclear Engineering (ICONE-3), p.0 - 0, 1995/00
None
Tateishi, Yoshinori*; Tanaka, Yasumasa; *; *; Goto, Tatsuro*; *; *
PNC TN841 84-66, 197 Pages, 1983/08
no abstracts in English
Miura, Makoto; Omori, Takuro; Sawayama, Yukio; Shiina, Sadamu; Tanaka, Yasumasa
PNC TN841 79-23, 77 Pages, 1979/03
no abstracts in English
Miura, Makoto; ; ; Omori, Takuro; ; Tanaka, Yasumasa; Shiina, Sadamu; Ogasawara, Koji
PNC TN841 79-12, 103 Pages, 1979/03
no abstracts in English
Miura, Makoto; Honda, Yutaka*; Omori, Takuro; Kaneko, Hiromitsu; ; Yato, Tadao*; Tanaka, Yasumasa; Konashi, Kenji
PNC TN841 79-11, 118 Pages, 1979/02
no abstracts in English