Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the  Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10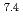 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:7 Percentile:79.28(Chemistry, Physical)no abstracts in English
Yasue, Ayumu*; Kobayashi, Kensuke*; Yoshioka, Masahiro*; Noma, Takashi*; Okuno, Koichi*; Tanaka, Seiichiro*; Hirata, Yoshikazu*; Ooka, Tokunao*; Kimura, Yoshiharu*; Nagai, Tomoya*; et al.
Journal of Advanced Concrete Technology, 21(5), p.337 - 350, 2023/05
Times Cited Count:0 Percentile:0.00(Construction & Building Technology)The purpose of this study was to evaluate the use of resin injection to repair cracks in ultra-high-strength concrete (UHSC) members. As a preliminary step, the applicability of the neutron diffraction method (NDM) to investigate the effect of repairs in UHSC specimens was examined. The experimental results showed that the NDM can measure stresses in rebars in UHSC and normal concrete specimens. Therefore, in this experiment, the NDM was used to measure the bond performance of repairs with epoxy resin around the slit in normal concrete and UHSC specimens and examine the effect of repair on the UHSC specimens. Displacement around the slit was measured using a PI-shape displacement transducer. The evaluation confirmed that the bond performance of the repaired area was recovered by resin injection regardless of the concrete strength. In addition, the displacement around the slit was smaller for the injected specimens than the noninjected specimens. These experimental results clarified that by injecting resin, the same bond repair effect could be obtained in UHSC and normal concrete specimens.

 -, SeO
-, SeO
 -, and SeO
-, and SeO
 -coprecipitated barite after treatment with phosphate
-coprecipitated barite after treatment with phosphateTokunaga, Kohei; Tanaka, Kazuya; Takahashi, Yoshio*; Kozai, Naofumi
Environmental Science & Technology, 57(8), p.3166 - 3175, 2023/02
Times Cited Count:4 Percentile:41.75(Engineering, Environmental)Coprecipitation of radionuclides with barite has been studied to remove radionuclides from radioactive liquid waste because of its excellent removal efficiency; however, little information exists concerning the stability of the ions coprecipitated with barite. This study systematically investigated the stability of iodate, selenite, and selenate coprecipitated with barite via leaching tests. These oxyanions were gradually leached from the oxyanion-bearing barite into ultrapure water over time. Leaching of the oxyanions significantly increased in leaching solutions containing NaCl (pH5.3), NaNO (pH5.9), and Na
(pH5.9), and Na SO
SO (pH5.7). Conversely, leaching of the oxyanions was suppressed in KH
(pH5.7). Conversely, leaching of the oxyanions was suppressed in KH PO
PO solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO
solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.
was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.
 molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:18 Percentile:68.88(Multidisciplinary Sciences)no abstracts in English
Shiraishi, Fumito*; Chihara, Ryoji*; Tanimoto, Risa*; Tanaka, Kazuya; Takahashi, Yoshio*
Island Arc, 31(1), p.e12448_1 - e12448_9, 2022/05
Times Cited Count:0 Percentile:0.00(Geosciences, Multidisciplinary)At Yunotaki Fall in north Japan, manganese-oxidizing bacteria were previously assumed to have oxidized manganese to precipitate birnessite, which relied on oxygen released from algae. However, it remained unclear whether larger-scale manganese oxide precipitation was actually occurring under light conditions. This study evaluated the contribution of indirect oxidation using microelectrodes to analyze local water chemistry, in addition to bulk water chemistry and DNA analyses. The results of this study demonstrate that low bulk pH values in the hot spring water hindered indirect oxidation despite the occurrence of active oxygenic photosynthesis and that direct oxidation by manganese-oxidizing bacteria is considered to dominate in the investigated sample.
Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
Nishimura, Shoichiro*; Torii, Hiroyuki*; Fukao, Yoshinori*; Ito, Takashi; Iwasaki, Masahiko*; Kanda, Sotaro*; Kawagoe, Kiyotomo*; Kawall, D.*; Kawamura, Naritoshi*; Kurosawa, Noriyuki*; et al.
Physical Review A, 104(2), p.L020801_1 - L020801_6, 2021/08
Times Cited Count:19 Percentile:83.44(Optics)Tokunaga, Kohei; Takahashi, Yoshio*; Tanaka, Kazuya; Kozai, Naofumi
Chemosphere, 266, p.129104_1 - 129104_10, 2021/03
Times Cited Count:15 Percentile:58.47(Environmental Sciences)Radioactive iodine ( I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
 ) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO
) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO ). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl
). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl concentration of 10 mmol L
concentration of 10 mmol L . Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
. Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
 substitution in SO
substitution in SO
 site is achieved by the substitution of Na
site is achieved by the substitution of Na -IO
-IO
 pairs at the nearest Ba
pairs at the nearest Ba site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Takagi, Yasuhiko*; Nakamura, Tomoki*; Hiroi, Takahiro*; Matsuoka, Moe*; et al.
Nature Astronomy (Internet), 5(3), p.246 - 250, 2021/03
Times Cited Count:60 Percentile:96.18(Astronomy & Astrophysics)Here we report observations of Ryugu's subsurface material by the Near-Infrared Spectrometer (NIRS3) on the Hayabusa2 spacecraft. Reflectance spectra of excavated material exhibit a hydroxyl (OH) absorption feature that is slightly stronger and peak-shifted compared with that observed for the surface, indicating that space weathering and/or radiative heating have caused subtle spectral changes in the uppermost surface. However, the strength and shape of the OH feature still suggests that the subsurface material experienced heating above 300  C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200
C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200  C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
Tanaka, Kazuya; Kanasashi, Tsutomu*; Takenaka, Chisato*; Takahashi, Yoshio*
Science of the Total Environment, 755, Part 2, p.142598_1 - 142598_8, 2021/02
Times Cited Count:5 Percentile:21.63(Environmental Sciences)In this study, we investigated coordination structures of Cs in  Cs-doped bark, sapwood, heartwood, needle, and branch samples of trees collected in Fukushima by extended X-ray absorption fine structure (EXAFS) spectroscopy. We examined four representative tree species in Fukushima,
Cs-doped bark, sapwood, heartwood, needle, and branch samples of trees collected in Fukushima by extended X-ray absorption fine structure (EXAFS) spectroscopy. We examined four representative tree species in Fukushima, 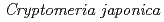 ,
, 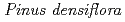 ,
, 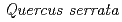 , and
, and 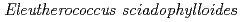 . EXAFS spectra suggested that Cs was adsorbed as an outer-sphere complex on all parts of the four species, with electrostatic binding to negatively charged functional groups in components of tree tissues. These results were supported by extraction experiments where most of the sorbed Cs was desorbed from all parts of each tree species using 1 M CH
. EXAFS spectra suggested that Cs was adsorbed as an outer-sphere complex on all parts of the four species, with electrostatic binding to negatively charged functional groups in components of tree tissues. These results were supported by extraction experiments where most of the sorbed Cs was desorbed from all parts of each tree species using 1 M CH COONH
COONH .
.
Rodriguez, G.*; Varaine, F.*; Costes, L.*; Venard, C.*; Serre, F.*; Chanteclair, F.*; Chenaud, M.-S.*; Dechelette, F.*; Hourcade, E.*; Plancq, D.*; et al.
EPJ Nuclear Sciences & Technologies (Internet), 7, p.15_1 - 15_8, 2021/00
France (CEA and FRAMATOME) and Japan (JAEA, MHI and MFBR) have carried out studies to establish a common technical view regarding sodium-cooled fast reactor concept. Japan and France performed a common work to examine ways to develop a feasible common design concept, which could be built both in France and/or in Japan. This paper is providing a review of this joint synthesis on Sodium Fast Reactor design concept.
Takahashi, Yoshio*; Sakaguchi, Aya*; Fan, Q.*; Tanaka, Kazuya; Miura, Hikaru*; Kurihara, Yuichi*
Behavior of Radionuclides in the Environment I; Function of Particles in Aquatic System, p.115 - 150, 2020/00
Times Cited Count:0 Percentile:0.00(Environmental Sciences)Abe, Mitsushi*; Bae, S.*; Beer, G.*; Bunce, G.*; Choi, H.*; Choi, S.*; Chung, M.*; da Silva, W.*; Eidelman, S.*; Finger, M.*; et al.
Progress of Theoretical and Experimental Physics (Internet), 2019(5), p.053C02_1 - 053C02_22, 2019/05
Times Cited Count:161 Percentile:99.30(Physics, Multidisciplinary)This paper introduces a new approach to measure the muon magnetic moment anomaly 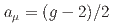 and the muon electric dipole moment (EDM)
and the muon electric dipole moment (EDM) 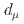 at the J-PARC muon facility. The goal of our experiment is to measure
at the J-PARC muon facility. The goal of our experiment is to measure 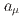 and
and 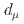 using an independent method with a factor of 10 lower muon momentum, and a factor of 20 smaller diameter storage-ring solenoid compared with previous and ongoing muon g-2 experiments with unprecedented quality of the storage magnetic field. Additional significant differences from the present experimental method include a factor of 1000 smaller transverse emittance of the muon beam (reaccelerated thermal muon beam), its efficient vertical injection into the solenoid, and tracking each decay positron from muon decay to obtain its momentum vector. The precision goal for
using an independent method with a factor of 10 lower muon momentum, and a factor of 20 smaller diameter storage-ring solenoid compared with previous and ongoing muon g-2 experiments with unprecedented quality of the storage magnetic field. Additional significant differences from the present experimental method include a factor of 1000 smaller transverse emittance of the muon beam (reaccelerated thermal muon beam), its efficient vertical injection into the solenoid, and tracking each decay positron from muon decay to obtain its momentum vector. The precision goal for 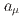 is a statistical uncertainty of 450 parts per billion (ppb), similar to the present experimental uncertainty, and a systematic uncertainty less than 70 ppb. The goal for EDM is a sensitivity of
is a statistical uncertainty of 450 parts per billion (ppb), similar to the present experimental uncertainty, and a systematic uncertainty less than 70 ppb. The goal for EDM is a sensitivity of 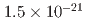 e
e cm.
cm.
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Arai, Takehiko*; Nakauchi, Yusuke*; Nakamura, Tomoki*; Matsuoka, Moe*; et al.
Science, 364(6437), p.272 - 275, 2019/04
Times Cited Count:303 Percentile:99.67(Multidisciplinary Sciences)The near-Earth asteroid 162173 Ryugu, the target of Hayabusa2 sample return mission, is believed to be a primitive carbonaceous object. The Near Infrared Spectrometer (NIRS3) on Hayabusa2 acquired reflectance spectra of Ryugu's surface to provide direct measurements of the surface composition and geological context for the returned samples. A weak, narrow absorption feature centered at 2.72 micron was detected across the entire observed surface, indicating that hydroxyl (OH)-bearing minerals are ubiquitous there. The intensity of the OH feature and low albedo are similar to thermally- and/or shock-metamorphosed carbonaceous chondrite meteorites. There are few variations in the OH-band position, consistent with Ryugu being a compositionally homogeneous rubble-pile object generated from impact fragments of an undifferentiated aqueously altered parent body.
Strasser, P.*; Abe, Mitsushi*; Aoki, Masaharu*; Choi, S.*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; et al.
EPJ Web of Conferences, 198, p.00003_1 - 00003_8, 2019/01
Times Cited Count:16 Percentile:98.61(Quantum Science & Technology)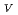 -edge XANES to determination of U oxidation state in zircon
-edge XANES to determination of U oxidation state in zirconTanaka, Kazuya; Takahashi, Yoshio*
Geochemical Journal, 53(5), p.329 - 331, 2019/00
Times Cited Count:1 Percentile:4.75(Geochemistry & Geophysics)We examined three natural zircon samples with different amounts of radiation doses using M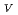 -edge and L
-edge and L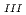 -edge U X-ray absorption near-edge structure (XANES). Analysis of XANES spectra at both M
-edge U X-ray absorption near-edge structure (XANES). Analysis of XANES spectra at both M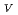 -edge and L
-edge and L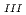 -edge suggested that the oxidation state of U in the zircon sample with the highest radiation dose is tetravalent. The XANES spectra of the two other samples with lower radiation doses suggested a mixture of U(IV) and U(VI), while the possibility of U(V) was not excluded. This is the first work on the application of M
-edge suggested that the oxidation state of U in the zircon sample with the highest radiation dose is tetravalent. The XANES spectra of the two other samples with lower radiation doses suggested a mixture of U(IV) and U(VI), while the possibility of U(V) was not excluded. This is the first work on the application of M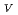 -edge U XANES to the oxidation state of U in natural zircon.
-edge U XANES to the oxidation state of U in natural zircon.
Sakaguchi, Aya*; Chiga, Haruka*; Tanaka, Kazuya; Tsuruta, Haruo*; Takahashi, Yoshio*
Geochemical Journal, 52(2), p.187 - 199, 2018/00
Times Cited Count:8 Percentile:33.75(Geochemistry & Geophysics)An aerosol sample collected on the 15th of March 2011 at Kawasaki City (Kanagawa) was sequentially leached with seawater for 30 days. As a result, about 60% of the total  Cs was extracted. In addition, a surface soil sample collected from Kawamata Town (Fukushima) two months after the Fukushima accident, was leached for 223 days with a natural seawater, a 1:1 mixture of ultrapure water and seawater, and ultrapure water. Eventually, more than 15% of the total
Cs was extracted. In addition, a surface soil sample collected from Kawamata Town (Fukushima) two months after the Fukushima accident, was leached for 223 days with a natural seawater, a 1:1 mixture of ultrapure water and seawater, and ultrapure water. Eventually, more than 15% of the total  Cs in the surface soil sample was efficiently desorbed by seawater leaching. In comparison, about 9% of the total
Cs in the surface soil sample was efficiently desorbed by seawater leaching. In comparison, about 9% of the total  Cs was leached with 1:1 diluted seawater and less than 1% of the total
Cs was leached with 1:1 diluted seawater and less than 1% of the total  Cs was leached with ultrapure water over the 223 days. Overall,
Cs was leached with ultrapure water over the 223 days. Overall,  Cs and
Cs and  Cs showed similar leaching behaviour.
Cs showed similar leaching behaviour.
Yamaguchi, Akiko*; Honda, Tasuku*; Tanaka, Masato*; Tanaka, Kazuya; Takahashi, Yoshio*
Geochemical Journal, 52(5), p.415 - 425, 2018/00
Times Cited Count:24 Percentile:70.82(Geochemistry & Geophysics)Ion-adsorption type REE deposits in weathered granite are main sources of REE essential for high-technology industries. However, these type deposits have not been searched in Japan. In this study, Bulk REE abundances (= REE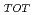 ), ion-exchangeable REE (= REE
), ion-exchangeable REE (= REE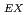 ) by ammonium chloride solution, and percentage of REE
) by ammonium chloride solution, and percentage of REE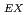 relative to REE
relative to REE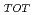 (= REE
(= REE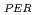 ) in fresh and weathered granite samples in Southwest Japan (i.e., Hiroshima and Shimane Prefectures) were determined. The REE
) in fresh and weathered granite samples in Southwest Japan (i.e., Hiroshima and Shimane Prefectures) were determined. The REE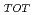 and REE
and REE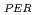 were comparable to those of ion-adsorption type REE deposits in China. Extended X-ray adsorption fine structure (EXAFS) spectra of REE in original samples and samples after the extraction of ion-exchangeable REE showed that (i) REE in samples with high REE
were comparable to those of ion-adsorption type REE deposits in China. Extended X-ray adsorption fine structure (EXAFS) spectra of REE in original samples and samples after the extraction of ion-exchangeable REE showed that (i) REE in samples with high REE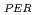 mainly forms outer-sphere complexes and (ii) the remaining REE in the rocks after the extraction forms inter-sphere complexes.
mainly forms outer-sphere complexes and (ii) the remaining REE in the rocks after the extraction forms inter-sphere complexes.
 ) of radiocesium in the Kuchibuto River in Fukushima
) of radiocesium in the Kuchibuto River in FukushimaMiura, Hikaru*; Kurihara, Yuichi*; Sakaguchi, Aya*; Tanaka, Kazuya; Yamaguchi, Noriko*; Higaki, Shogo*; Takahashi, Yoshio*
Geochemical Journal, 52(2), p.145 - 154, 2018/00
Times Cited Count:55 Percentile:92.80(Geochemistry & Geophysics)Solid-water distribution coefficient ( ) of radiocesium in rivers is apparently increased due to the possible presence of highly radioactive radiocesium-bearing microparticles (CsMPs) in the solid phase. In this study, we evaluated the contribution of CsMPs to apparent Kd values. The ratio of the radioactivity of the separated CsMPs to the total radiocesium on fluvial suspended particles ranged from 0 to 46%. This means that the existence of CsMPs in fluvial suspended partcles did not change apparent Kd values in order magnitude.
) of radiocesium in rivers is apparently increased due to the possible presence of highly radioactive radiocesium-bearing microparticles (CsMPs) in the solid phase. In this study, we evaluated the contribution of CsMPs to apparent Kd values. The ratio of the radioactivity of the separated CsMPs to the total radiocesium on fluvial suspended particles ranged from 0 to 46%. This means that the existence of CsMPs in fluvial suspended partcles did not change apparent Kd values in order magnitude.