Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Watanabe, So; Takahatake, Yoko; Ogi, Hiromichi*; Osugi, Takeshi; Taniguchi, Takumi; Sato, Junya; Arai, Tsuyoshi*; Kajinami, Akihiko*
Journal of Nuclear Materials, 585, p.154610_1 - 154610_6, 2023/11
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Kataoka, Noriyuki*; Tanaka, Masashi*; Hosoda, Wataru*; Taniguchi, Takumi*; Fujimori, Shinichi; Wakita, Takanori*; Muraoka, Yuji*; Yokoya, Takashi*
Journal of Physics; Condensed Matter, 33(3), p.035501_1 - 035501_6, 2021/01
Times Cited Count:4 Percentile:33.7(Physics, Condensed Matter)Irisawa, Keita; Kudo, Isamu*; Taniguchi, Takumi; Namiki, Masahiro*; Osugi, Takeshi; Nakazawa, Osamu
QST-M-16; QST Takasaki Annual Report 2017, P. 63, 2019/03
no abstracts in English
Garcia-Lodeiro, I.*; Lebon, R.*; Machoney, D.*; Zhang, B.*; Irisawa, Keita; Taniguchi, Takumi; Namiki, Masahiro*; Osugi, Takeshi; Meguro, Yoshihiro; Kinoshita, Hajime*
Proceedings of 3rd International Symposium on Cement-based Materials for Nuclear Wastes (NUWCEM 2018) (USB Flash Drive), 4 Pages, 2018/11
 O in Fe
O in Fe O
O film on Fe formed in an NaOH solution containing oxidants
film on Fe formed in an NaOH solution containing oxidantsHaruna, Takumi*; Miyataki, Yuki*; Hirohata, Yohei*; Shibata, Toshio*; Taniguchi, Naoki; Tachikawa, Hirokazu*
Zairyo To Kankyo, 67(9), p.375 - 380, 2018/09
This research aimed to confirm the formation of Fe O
O film on Fe immersed in aqueous 45 mass% NaOH solution containing some oxidants at the boiling temperature, to recognize the optimum immersion time for the formation of thick and protective film, and to reveal the absorption behavior of D
film on Fe immersed in aqueous 45 mass% NaOH solution containing some oxidants at the boiling temperature, to recognize the optimum immersion time for the formation of thick and protective film, and to reveal the absorption behavior of D O in the Fe
O in the Fe O
O film at room temperature. The results were obtained as follows. It was confirmed that Fe
film at room temperature. The results were obtained as follows. It was confirmed that Fe O
O film was formed on Fe immersed in the NaOH solution for a time more than 0.6 ks, and the film thickness increased parabolically with an increase in the immersion time. D
film was formed on Fe immersed in the NaOH solution for a time more than 0.6 ks, and the film thickness increased parabolically with an increase in the immersion time. D O absorption test was carried out to the films formed in the NaOH solution for immersion times of 1.2 and 3.6 ks. An amount of D
O absorption test was carried out to the films formed in the NaOH solution for immersion times of 1.2 and 3.6 ks. An amount of D O absorbed into the film increased with an increase in an absorption time up to 1000 ks, and an absorption time more than 1000 ks made an amount of D
O absorbed into the film increased with an increase in an absorption time up to 1000 ks, and an absorption time more than 1000 ks made an amount of D O constant. The constant amount of D
O constant. The constant amount of D O was larger for the film formed on Fe immersed in the NaOH solution for 3.6 ks than that for 1.2 ks. The transient of the amount of D
O was larger for the film formed on Fe immersed in the NaOH solution for 3.6 ks than that for 1.2 ks. The transient of the amount of D O absorbed into the film was analyzed on the basis of Fick's law for diffusion, and diffusion coefficients of D
O absorbed into the film was analyzed on the basis of Fick's law for diffusion, and diffusion coefficients of D O were obtained to be 5.1
O were obtained to be 5.1 10
10 cm
cm s
s and 9.9
and 9.9 10
10 cm
cm s
s for the films formed for 1.2 and 3.6 ks, respectively. Therefore it was estimated that the diffusion coefficient of the Fe
for the films formed for 1.2 and 3.6 ks, respectively. Therefore it was estimated that the diffusion coefficient of the Fe O
O film was in the region from 5.1
film was in the region from 5.1 10
10 cm
cm s
s to 9.9
to 9.9 10
10 cm
cm s
s .
.
 -ray during dehydration
-ray during dehydrationIrisawa, Keita; Kudo, Isamu*; Taniguchi, Takumi; Namiki, Masahiro*; Osugi, Takeshi; Nakazawa, Osamu
QST-M-8; QST Takasaki Annual Report 2016, P. 63, 2018/03
A solidification technique with minimized water content is being developed using a phosphate cement for safe storage of secondary radioactive wastes in the Fukushima Daiichi Nuclear Power Plant. To understand the applicability of the solidification technique for the actual secondary wastes, phosphate cement during dehydration was irradiated by  Co
Co  -ray. The G(H
-ray. The G(H ) for the phosphate cement decreased with time during dehydration, and was not detected after 7 days. Moreover, the
) for the phosphate cement decreased with time during dehydration, and was not detected after 7 days. Moreover, the  Co
Co  -ray irradiation during dehydration did not change the crystalline and amorphous phases of the phosphate cement.
-ray irradiation during dehydration did not change the crystalline and amorphous phases of the phosphate cement.
Kato, Jun; Nakagawa, Akinori; Taniguchi, Takumi; Sakakibara, Tetsuro; Nakazawa, Osamu; Meguro, Yoshihiro
JAEA-Review 2017-015, 173 Pages, 2017/07
Various radioactive wastes have been generated at the Fukushima Daiichi Nuclear Power Station (1F). To dispose of the wastes underground, it is necessary to make a suitable waste package by the volume reduction and solidification of the wastes. To plan the future decommissioning of 1F, it is also necessary to estimate feasibility of existing treatment technology for those wastes. Therefore the document survey has been performed about volume reduction and solidification technologies that have domestic or foreign experiences of practical treatment for radioactive wastes to assist selection of suitable treatment of the wastes. This report shows the arranged results. The 1F wastes are classified into two groups, homogeneous particulate and liquid wastes and heterogeneous solid wastes. The needful items for the feasibility study such as a technology name, a fundamental principle, treatment efficiency, and characteristic of solidified waste are summarized in each group.
Irisawa, Keita; Taniguchi, Takumi; Namiki, Masahiro; Garc a-Lodeiro, I.*; Osugi, Takeshi; Sakakibara, Tetsuro; Nakazawa, Osamu; Meguro, Yoshihiro; Kinoshita, Hajime*
a-Lodeiro, I.*; Osugi, Takeshi; Sakakibara, Tetsuro; Nakazawa, Osamu; Meguro, Yoshihiro; Kinoshita, Hajime*
Proceedings of 2017 International Congress on Advances in Nuclear Power Plants (ICAPP 2017) (CD-ROM), 6 Pages, 2017/04
A solidification technique with minimized water content is being developed using phosphate cements for the safe storage of secondary radioactive wastes in the Fukushima Daiichi Nuclear Power Plant. Conventional cement systems become solidified via hydration reactions, and need a certain water content. Phosphate cement systems, however, become solidified via an acid-base reaction, and so they only require water mainly for reasons of workability. A reduced water content of phosphate cement systems is beneficial for the immobilization of the radioactive wastes from mitigating the potential to generate hydrogen gas by the radiolysis of water by radioactive wastes. The current study investigated the water content and mineralogy of calcium aluminate cement (CAC) and phosphate-modified CAC (CAP) cured in open systems at 60, 90 and 120  C and in a closed system at 20
C and in a closed system at 20  C as a reference case. Water contents in both the CAC and the CAP were seen to decrease as curing progressed. For
C as a reference case. Water contents in both the CAC and the CAP were seen to decrease as curing progressed. For  90
90  C, the CAP contained less water than CAC. Free water in CAC converted to structural water by heat treatment, but this was not the case for CAP. An orthophosphate hydrate salt, a precursor phase of hydroxyapatite, was found in CAP when cured at 20 and 60
C, the CAP contained less water than CAC. Free water in CAC converted to structural water by heat treatment, but this was not the case for CAP. An orthophosphate hydrate salt, a precursor phase of hydroxyapatite, was found in CAP when cured at 20 and 60  C, and a mixture of the orthophosphate hydrate salt and hydroxyapatite, Ca
C, and a mixture of the orthophosphate hydrate salt and hydroxyapatite, Ca (PO
(PO )
) (OH)
(OH) , were formed in the CAP when cured at 90
, were formed in the CAP when cured at 90  C. Phosphate products in CAP cured at 120
C. Phosphate products in CAP cured at 120  C appears to consist of a different phosphate phase compared with the CAP cured at 20, 60 and 90
C appears to consist of a different phosphate phase compared with the CAP cured at 20, 60 and 90  C.
C.
Taniguchi, Takumi; Abe, Tomohisa
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 23(1), p.73 - 74, 2016/06
The weekend basic course for Division of Nuclear Fuel Cycle and Environment was held on November 7 (Sat) and 8 (Sun) on 2015 at Tohoku University Aobayama Campus in Sendai City. Twenty-eight people from universities and companies attended the course, and seven lectures and group discussions were held. I will report the outline of this course and the contents of the group discussion.
 O in the film on Fe oxidized at high temperature in air
O in the film on Fe oxidized at high temperature in airHaruna, Takumi*; Yamamoto, Tatsuya*; Miyairi, Yoji*; Shibata, Toshio*; Taniguchi, Naoki; Sakamaki, Keiko; Tachikawa, Hirokazu*
Zairyo To Kankyo, 64(5), p.201 - 206, 2015/05
Diffusion coefficients of D O in the films was determined in order to estimate corrosion rate of carbon steel for the overpack in ground water. Fe plates were heated to form oxide films. The films were characterized with XRD and SEM. After that, the specimen was contacted with D
O in the films was determined in order to estimate corrosion rate of carbon steel for the overpack in ground water. Fe plates were heated to form oxide films. The films were characterized with XRD and SEM. After that, the specimen was contacted with D O for 5184 ks, followed by subjected to TDS to obtain an amount of D
O for 5184 ks, followed by subjected to TDS to obtain an amount of D O absorbing into the film. As a result, single-layered film of Fe
O absorbing into the film. As a result, single-layered film of Fe O
O was formed at 573 and 723 K, and double-layered film of Fe
was formed at 573 and 723 K, and double-layered film of Fe O
O and Fe
and Fe O
O was formed at 873 K. It was found that an amount of D
was formed at 873 K. It was found that an amount of D O in the film correlated linearly with a square root of the absorption period, and that the amount was steady for a long period. From the results and Fick's second law, diffusion coefficients of D
O in the film correlated linearly with a square root of the absorption period, and that the amount was steady for a long period. From the results and Fick's second law, diffusion coefficients of D O was determined as 9.7
O was determined as 9.7 10
10 cm
cm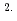 s
s for the Fe
for the Fe O
O film, and 5.5
film, and 5.5 10
10 cm
cm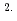 s
s to 2.2
to 2.2 10
10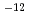 cm
cm s
s for Fe
for Fe O
O film.
film.
 Rn-
Rn- At generator
At generatorMaeda, Eita*; Yokoyama, Akihiko*; Taniguchi, Takumi*; Washiyama, Koshin*; Nishinaka, Ichiro
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1465 - 1468, 2015/02
Times Cited Count:9 Percentile:60.64(Chemistry, Analytical)The  At isotope has gathered attention as a promising
At isotope has gathered attention as a promising  -emitter for radionuclide therapy. We report the dependence of the distribution ratio of astatine on the concentration of HCl, and on the polarity of the organic solvent. The results will be useful for development of the
-emitter for radionuclide therapy. We report the dependence of the distribution ratio of astatine on the concentration of HCl, and on the polarity of the organic solvent. The results will be useful for development of the  Rn-
Rn- At generator.
At generator.
 X-ray diffraction of graphite-diamond transformation using various catalysts under high pressures and high temperatures
X-ray diffraction of graphite-diamond transformation using various catalysts under high pressures and high temperaturesUtsumi, Wataru; Okada, Taku; Taniguchi, Takashi*; Funakoshi, Kenichi*; Kikegawa, Takumi*; Hamaya, Nozomu; Shimomura, Osamu
Journal of Physics; Condensed Matter, 16(14), p.S1017 - S1026, 2004/04
Times Cited Count:14 Percentile:54(Physics, Condensed Matter)The graphite-diamond transformation was investigated using in-situ time-resolved X-ray diffraction experiments with a MgO dissolved aqueous fluid as the diamond forming catalyst under conditions of 6.6-8.8 GPa and 1400-1835 C. Experiments were conducted using a 180-ton DIA-type cubic-anvil apparatus installed on the beamline BL14B1 at SPring-8, a third-generation synchrotron radiation facility in Japan. By analyzing the kinetic data using the JMAK rate equation, it was clarified that altering the pressure-temperature conditions drastically changes the nucleation and growth process of diamond.
C. Experiments were conducted using a 180-ton DIA-type cubic-anvil apparatus installed on the beamline BL14B1 at SPring-8, a third-generation synchrotron radiation facility in Japan. By analyzing the kinetic data using the JMAK rate equation, it was clarified that altering the pressure-temperature conditions drastically changes the nucleation and growth process of diamond.
Inoue, Takashi; Hanada, Masaya; Iga, Takashi*; Imai, Tsuyoshi; Kashiwagi, Mieko; Kawai, Mikito; Morishita, Takatoshi; Taniguchi, Masaki; Umeda, Naotaka; Watanabe, Kazuhiro; et al.
Fusion Engineering and Design, 66-68, p.597 - 602, 2003/09
Times Cited Count:21 Percentile:78.49(Nuclear Science & Technology)The neutral beam (NB) injection has been one of the most promising methods for plasma heating and current drive in tokamak fusion devices. JAERI has developed high energy electrostatic accelerators for the NB systems in JT-60U and ITER. Recent progress on this R&D are as follows: 1) In the JT-60U NB system, some of the beams has been deflected due to distorted electric field in the accelerator, resulting in an excess heat load on the NB port. By correcting the electric field, a continuous injection of H beam was succeeded for 10 s with the NB power of 2.6 MW at 355 keV. 2) To increase the beam energy, a metal structure called stress ring was designed. The ring reduces electric field concentration at the triple junction point (interface between metal and dielectric insulator inside vacuum). Initial test of the accelerators with the stress rings has shown higher voltage hold off performance in both accelerators for JT-60U and ITER R&D than that without rings.
beam was succeeded for 10 s with the NB power of 2.6 MW at 355 keV. 2) To increase the beam energy, a metal structure called stress ring was designed. The ring reduces electric field concentration at the triple junction point (interface between metal and dielectric insulator inside vacuum). Initial test of the accelerators with the stress rings has shown higher voltage hold off performance in both accelerators for JT-60U and ITER R&D than that without rings.
Taniguchi, Takashi*; Sato, Masashi*; Utsumi, Wataru; Kikegawa, Takumi*; Shimomura, Osamu
Diamond and Related Materials, 6(12), p.1806 - 1815, 1997/12
Times Cited Count:17 Percentile:69.66(Materials Science, Multidisciplinary)no abstracts in English
Taniguchi, Takashi*; Sato, Masashi*; Utsumi, Wataru; Kikegawa, Takumi*; Shimomura, Osamu
Applied Physics Letters, 70(18), p.2392 - 2394, 1997/05
Times Cited Count:25 Percentile:74.65(Physics, Applied)no abstracts in English
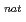 Pb reaction; Aim at the new cancer medical treatment by
Pb reaction; Aim at the new cancer medical treatment by  -emitting radioisotopes
-emitting radioisotopesNishinaka, Ichiro; Makii, Hiroyuki; Toyoshima, Atsushi; Yokoyama, Akihiko*; Washiyama, Koshin*; Amano, Ryohei*; Maeda, Eita*; Yamada, Norihiro*; Taniguchi, Takumi*; Watanabe, Shigeki; et al.
no journal, ,
no abstracts in English
 Li ion beams
Li ion beamsNishinaka, Ichiro; Yokoyama, Akihiko*; Washiyama, Koshin*; Amano, Ryohei*; Maeda, Eita*; Taniguchi, Takumi*; Murakami, Kento*; Watanabe, Shigeki; Suzuki, Hiroyuki; Ishioka, Noriko; et al.
no journal, ,
In general, an  -emitter
-emitter  At which is a prospective candidate for utilization in targeted alpha radiotherapy is produced through the
At which is a prospective candidate for utilization in targeted alpha radiotherapy is produced through the  Bi(
Bi( He, 2n)
He, 2n) At reaction. In contrast, our project is focusing on the production in the
At reaction. In contrast, our project is focusing on the production in the  Bi(
Bi( Li, 5n)
Li, 5n) Rn reaction. This enables us to supply
Rn reaction. This enables us to supply  At in a
At in a  Rn/
Rn/ At generator system. The daughter
At generator system. The daughter  At (7.2 h half-life) is extracted from the parent
At (7.2 h half-life) is extracted from the parent  Rn (14h), expanding time-frame for transportation and use of
Rn (14h), expanding time-frame for transportation and use of  At. To use astatine and iodine radioisotopes in our project, the excitation functions of
At. To use astatine and iodine radioisotopes in our project, the excitation functions of 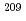 Bi(
Bi( Li, xn)
Li, xn)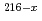 Rn,
Rn, 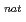 Pb(
Pb( Li, xn)
Li, xn)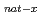 At and
At and 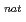 Sn(
Sn( Li, xn)
Li, xn)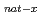 I reactions have been measured. In addition to that, separation techniques have been developed. We report not only on the production and separation of astatine and iodine radioisotopes in the reactions but also on the utilization of those radioisotopes.
I reactions have been measured. In addition to that, separation techniques have been developed. We report not only on the production and separation of astatine and iodine radioisotopes in the reactions but also on the utilization of those radioisotopes.
 Rn/
Rn/ At generator for targeted alpha therapy
At generator for targeted alpha therapyWashiyama, Koshin*; Maeda, Eita*; Yokoyama, Akihiko*; Nishinaka, Ichiro; Taniguchi, Takumi*; Yamada, Norihiro*; Makii, Hiroyuki; Toyoshima, Atsushi; Amano, Ryohei*
no journal, ,
A  Rn/
Rn/ At generator for targeted alpha therapy has been developed.
At generator for targeted alpha therapy has been developed.
Taniguchi, Takumi; Irisawa, Keita; Ito, Yuzuru; Namiki, Masahiro; Osugi, Takeshi; Abe, Tomohisa; Sato, Junya; Sakakibara, Tetsuro; Nakazawa, Osamu; Meguro, Yoshihiro; et al.
no journal, ,
no abstracts in English
Haruna, Takumi*; Miyataki, Yuki*; Hirohata, Yohei*; Taniguchi, Naoki; Tachikawa, Hirokazu*
no journal, ,
no abstracts in English