Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Takuro*; Fukuoka, Masafumi*; Toda, Kanako*; Nakanishi, Takahiro; Terashima, Motoki; Fujiwara, Kenso; Niwano, Yuma*; Kato, Hiroaki*; Kobayashi, Natsuko*; Tanoi, Keitaro*; et al.
ACS ES&T Water (Internet), 4(8), p.3579 - 3586, 2024/08
Nakagawa, Taichi; Suzuki, Reika*; Matsueda, Makoto; Terashima, Motoki; Hinze, W. L.*; Takagai, Yoshitaka*
Solvent Extraction Research and Development, Japan, 31(2), p.49 - 56, 2024/00
 Sr in human teeth and marine fish bones
Sr in human teeth and marine fish bonesKoarai, Kazuma; Matsueda, Makoto; Aoki, Jo; Terashima, Motoki
KEK Proceedings 2023-2, p.155 - 160, 2023/11
In this presentation, we report the results of the application of the Y-separation method using DGA resin to the determination of  Sr in human teeth and seawater fish bones.
Sr in human teeth and seawater fish bones.
Terashima, Motoki; Endo, Takashi*; Kimuro, Shingo; Beppu, Hikari*; Nemoto, Kazuaki*; Amano, Yuki
Journal of Nuclear Science and Technology, 60(4), p.374 - 384, 2023/04
Times Cited Count:4 Percentile:28.39(Nuclear Science & Technology) -CO
-CO mixed-gas reaction in inductively coupled plasma tandem quadrupole mass spectrometry
mixed-gas reaction in inductively coupled plasma tandem quadrupole mass spectrometryMatsueda, Makoto; Aoki, Jo; Koarai, Kazuma; Terashima, Motoki; Takagai, Yoshitaka*
Analytical Sciences, 38(11), p.1371 - 1376, 2022/11
Times Cited Count:8 Percentile:56.30(Chemistry, Analytical)The  I analysis using ICP-MS is challenging caused by xenon-129 (
I analysis using ICP-MS is challenging caused by xenon-129 ( Xe) and
Xe) and  IH
IH generated from excess stable isotope
generated from excess stable isotope  I. In this study, mass discrimination between iodine-129 (
I. In this study, mass discrimination between iodine-129 ( I) and interfering substances was achieved by inductively coupled plasma-tandem quadrupole mass spectrometry (ICP-MS/MS) with a dynamic reaction cell introduced a mixture gas of O
I) and interfering substances was achieved by inductively coupled plasma-tandem quadrupole mass spectrometry (ICP-MS/MS) with a dynamic reaction cell introduced a mixture gas of O and CO
and CO . As a result, the ratio of (background noise intensity at m/z 129)/
. As a result, the ratio of (background noise intensity at m/z 129)/ I was 3.8
I was 3.8  10
10 and 10 mBq/L of
and 10 mBq/L of  I was analyzed without chemical separation in the presence of 100 mg/L stable
I was analyzed without chemical separation in the presence of 100 mg/L stable  I. Spiked tests with actual rainwater were performed, and obtained values were agreed with the spiked amounts.
I. Spiked tests with actual rainwater were performed, and obtained values were agreed with the spiked amounts.
 Sr with ICP-MS/MS using O
Sr with ICP-MS/MS using O and NH
and NH mixed gas reaction
mixed gas reactionKoarai, Kazuma; Matsueda, Makoto; Terashima, Motoki
KEK Proceedings 2022-2, p.102 - 107, 2022/11
Analytical methods with inductively coupled plasm mass spectrometry (ICP-MS) have been developed for the determination of  Sr in environmental samples; however, the sensitivity of the ICP-MS methods and removal of interferences are insufficient to measure trace amount of
Sr in environmental samples; however, the sensitivity of the ICP-MS methods and removal of interferences are insufficient to measure trace amount of  Sr in the environmental samples. In this study, we developed an analytical method for
Sr in the environmental samples. In this study, we developed an analytical method for  Sr with ICP-DRC-MS/MS using oxygen and ammonia mixed gas reaction. This analytical method could be applied for measurement of
Sr with ICP-DRC-MS/MS using oxygen and ammonia mixed gas reaction. This analytical method could be applied for measurement of  Sr in reference soil.
Sr in reference soil.
Kuwata, Haruka*; Tazoe, Hirofumi*; Kranrod, C.*; Fujiwara, Kenso; Terashima, Motoki; Matsueda, Makoto; Hirao, Shigekazu*; Akata, Naofumi*
Radiation Protection Dosimetry, 198(13-15), p.1014 - 1018, 2022/09
Times Cited Count:0 Percentile:0.00(Environmental Sciences) Reactions to Achieve Mass-spectrometric Discrimination in Simultaneous Plutonium-isotope Speciation with Inductively Coupled Plasma-Tandem Mass Spectrometry
Reactions to Achieve Mass-spectrometric Discrimination in Simultaneous Plutonium-isotope Speciation with Inductively Coupled Plasma-Tandem Mass SpectrometryMatsueda, Makoto; Kawakami, Tomohiko*; Koarai, Kazuma; Terashima, Motoki; Fujiwara, Kenso; Iijima, Kazuki; Furukawa, Makoto*; Takagai, Yoshitaka*
Chemistry Letters, 51(7), p.678 - 682, 2022/07
Times Cited Count:11 Percentile:60.41(Chemistry, Multidisciplinary)New methodology for a simultaneous isotope speciation of various Pu isotopes without complicated isobaric interferences is developed by using inductively coupled plasma-mass spectrometry (ICP-MS). In analyzing ICP tandem MS (ICP-MS/MS), CO gas reactions in a dynamic reaction cell (DRC) almost eliminated the background noise intensity produced by isobaric interference from isotopes originating from actinides such as Am, Cm, and U at the locations (m/z) of significant Pu isotopes (
gas reactions in a dynamic reaction cell (DRC) almost eliminated the background noise intensity produced by isobaric interference from isotopes originating from actinides such as Am, Cm, and U at the locations (m/z) of significant Pu isotopes ( Pu,
Pu,  Pu,
Pu,  Pu,
Pu, 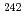 Pu,
Pu,  Pu).
Pu).
 Sr in hard tissue samples
Sr in hard tissue samplesKoarai, Kazuma; Matsueda, Makoto; Aoki, Jo; Yanagisawa, Kayo*; Fujiwara, Kenso; Terashima, Motoki
KEK Proceedings 2021-2, p.140 - 145, 2021/12
Strontium-90 and  Y, its daughter nuclide, adverse effects on the bone marrow. Monitoring of
Y, its daughter nuclide, adverse effects on the bone marrow. Monitoring of  Sr in the bones have been required after the Fukushima-Daiichi Nuclear Power Plant accident. However, conventional radioactivity measurement method for
Sr in the bones have been required after the Fukushima-Daiichi Nuclear Power Plant accident. However, conventional radioactivity measurement method for  Sr requires a complicated separation of
Sr requires a complicated separation of  Y and a time-consuming measurement. ICP-MS system has been applied to
Y and a time-consuming measurement. ICP-MS system has been applied to  Sr concentration survey of water, soil, and edible part of fish. We developed measurement method of
Sr concentration survey of water, soil, and edible part of fish. We developed measurement method of  Sr with ICP-MS and applied the method for cattle bones. We determined
Sr with ICP-MS and applied the method for cattle bones. We determined  Sr in the hard tissues of animals that collected in the Fukushima prefecture. Limit of detection in the measurement was 19 Bq/kg.
Sr in the hard tissues of animals that collected in the Fukushima prefecture. Limit of detection in the measurement was 19 Bq/kg.
 Sr in cattle bone and tooth samples by inductively coupled plasma mass spectrometry
Sr in cattle bone and tooth samples by inductively coupled plasma mass spectrometryKoarai, Kazuma; Matsueda, Makoto; Aoki, Jo; Yanagisawa, Kayo*; Terashima, Motoki; Fujiwara, Kenso; Kino, Yasushi*; Oka, Toshitaka; Takahashi, Atsushi*; Suzuki, Toshihiko*; et al.
Journal of Analytical Atomic Spectrometry, 36(8), p.1678 - 1682, 2021/08
Times Cited Count:7 Percentile:52.87(Chemistry, Analytical)Rapid analysis of  Sr in bone and tooth samples of cattle were achieved by an inductively coupled plasma mass spectrometry (ICP-MS) coupled with mass shift and solid phase extraction techniques. Limit of detection (LOD) in the ICP-MS measurement of 0.1 g samples was lower than that of the radioactivity measurement. Analytical time of the ICP-MS method was reduced from 20 days to 11 hours, compared with the radiometric method. Therefore, the ICP-MS method can be rapid and useful procedure of
Sr in bone and tooth samples of cattle were achieved by an inductively coupled plasma mass spectrometry (ICP-MS) coupled with mass shift and solid phase extraction techniques. Limit of detection (LOD) in the ICP-MS measurement of 0.1 g samples was lower than that of the radioactivity measurement. Analytical time of the ICP-MS method was reduced from 20 days to 11 hours, compared with the radiometric method. Therefore, the ICP-MS method can be rapid and useful procedure of  Sr in small bone and tooth samples derived from terrestrial animals.
Sr in small bone and tooth samples derived from terrestrial animals.
Matsueda, Makoto; Yanagisawa, Kayo*; Koarai, Kazuma; Terashima, Motoki; Fujiwara, Kenso; Abe, Hironobu; Kitamura, Akihiro; Takagai, Yoshitaka*
ACS Omega (Internet), 6(29), p.19281 - 19290, 2021/07
Times Cited Count:4 Percentile:19.51(Chemistry, Multidisciplinary)Online solid-phase extraction-inductively coupled plasma-quadrupole mass spectrometry with oxygen dynamic reaction cell (online SPE-ICP-MS-DRC) was shown to be a thorough automatic analytical system, circumventing the need for human handling. At three stepwise separations (SPE-DRC-Q mass filters), we showed that interference materials allowed the coexistence of abundance ratios of 1.5 10
10 for
for  Tc/Mo. Using this optimized system, a detection limit of
Tc/Mo. Using this optimized system, a detection limit of  Tc was 9.3 pg/L (5.9 mBq/L) for a 50 mL injection and sequential measurements were undertaken at a cycle of 24 min/sample.
Tc was 9.3 pg/L (5.9 mBq/L) for a 50 mL injection and sequential measurements were undertaken at a cycle of 24 min/sample.
Aoki, Jo; Matsueda, Makoto; Koarai, Kazuma; Terashima, Motoki; Fujiwara, Kenso; Abe, Hironobu
JAEA-Research 2021-002, 20 Pages, 2021/05
In order to analyze extremely low concentrations of  I in environmental samples by ICP-MS with high sensitivity and rapidity, it is necessary to remove interfering elements (Na, Mg, K, Ca, Mo, Cd and In) using a pretreatment method with Solid-phase Extraction Resin. Anion Exchange Resins with amino groups have been widely used as Solid-phase Extraction Resins, while Ag+ Supported Resins have also been widely used in recent years. It is necessary to optimize the pretreatment technique based on characteristics of the resins. In this study, we compared in terms of separation of I from matrix elements (Na, Mg, K, Ca, Mo, Cd and In) for DOWEX1-X8, AG 1-X8 and CL Resin, and investigated their suitability for ICP-MS analysis of
I in environmental samples by ICP-MS with high sensitivity and rapidity, it is necessary to remove interfering elements (Na, Mg, K, Ca, Mo, Cd and In) using a pretreatment method with Solid-phase Extraction Resin. Anion Exchange Resins with amino groups have been widely used as Solid-phase Extraction Resins, while Ag+ Supported Resins have also been widely used in recent years. It is necessary to optimize the pretreatment technique based on characteristics of the resins. In this study, we compared in terms of separation of I from matrix elements (Na, Mg, K, Ca, Mo, Cd and In) for DOWEX1-X8, AG 1-X8 and CL Resin, and investigated their suitability for ICP-MS analysis of  I in environmental samples. The results of adsorption and elution experiments showed that all resins examined uptake and elute I quantitatively. CL Resin showed the highest removal performance of interfering elements, with 3.1% of Mo remaining, but other interfering elements were removed below the detection limit of ICP-MS. However, the Mo remained after the CL Resin treatment could interfere the ICP-MS measurement of
I in environmental samples. The results of adsorption and elution experiments showed that all resins examined uptake and elute I quantitatively. CL Resin showed the highest removal performance of interfering elements, with 3.1% of Mo remaining, but other interfering elements were removed below the detection limit of ICP-MS. However, the Mo remained after the CL Resin treatment could interfere the ICP-MS measurement of  I, based on the consideration of ratio of
I, based on the consideration of ratio of  I and Mo. The eluate from CL Resin was treated with a Cation exchange resin (DOWEX 50WX8). As a result, Mo in the eluate was removed by up to 98% and the interference from Mo was reduced to measurable level. Therefore, the pretreatment method using CL Resin in combination with DOWEX 50WX8 is effective for ICP-MS analysis of
I and Mo. The eluate from CL Resin was treated with a Cation exchange resin (DOWEX 50WX8). As a result, Mo in the eluate was removed by up to 98% and the interference from Mo was reduced to measurable level. Therefore, the pretreatment method using CL Resin in combination with DOWEX 50WX8 is effective for ICP-MS analysis of  I at extremely low concentrations (background level).
I at extremely low concentrations (background level).
Endo, Takashi*; Tachi, Yukio; Ishidera, Takamitsu; Terashima, Motoki
Nihon Genshiryoku Gakkai Wabun Rombunshi, 20(1), p.9 - 22, 2021/03
Evaluation method of colloid diffusion and filtration in compacted bentonites was developed using dendrimers. Diffusion and filtration behavior of PAMAM dendrimers with the size of 5.7 7.2nm was investigated by the through-diffusion experiment in bentonite compacted to 0.8 Mg/m
7.2nm was investigated by the through-diffusion experiment in bentonite compacted to 0.8 Mg/m and saturated with 0.005
and saturated with 0.005 0.5mol/L NaCl. Effective diffusivities (De) and filtration ratios (Rf) of dendrimers were determined from the breakthrough curves and the depth profiles in compacted bentonite, respectively. The De values of negatively charged dendrimer increased when porewater salinity increased and dendrimer size decreased as influenced by anion exclusion effect in negatively charged clay surfaces. The Rf values increased when porewater salinity decreased and dendrimer size increased, demonstrating significant fractions of dendrimer were filtered by narrow pores in complex pore networks. These trends consistent with the previous studies emphasize the validity of the evaluation method using dendrimer.
0.5mol/L NaCl. Effective diffusivities (De) and filtration ratios (Rf) of dendrimers were determined from the breakthrough curves and the depth profiles in compacted bentonite, respectively. The De values of negatively charged dendrimer increased when porewater salinity increased and dendrimer size decreased as influenced by anion exclusion effect in negatively charged clay surfaces. The Rf values increased when porewater salinity decreased and dendrimer size increased, demonstrating significant fractions of dendrimer were filtered by narrow pores in complex pore networks. These trends consistent with the previous studies emphasize the validity of the evaluation method using dendrimer.
 Sr in hard tissue samples
Sr in hard tissue samplesKoarai, Kazuma; Matsueda, Makoto; Aoki, Jo; Yanagisawa, Kayo*; Fujiwara, Kenso; Terashima, Motoki; Kitamura, Akihiro; Abe, Hironobu
KEK Proceedings 2020-4, p.180 - 185, 2020/11
Strontium-90 and  Y, its daughter nuclide, adverse effects on the bone marrow. Monitoring of
Y, its daughter nuclide, adverse effects on the bone marrow. Monitoring of  Sr in the bones have been required after the Fukushima-Daiichi Nuclear Power Plant accident. However, conventional radioactivity measurement method for
Sr in the bones have been required after the Fukushima-Daiichi Nuclear Power Plant accident. However, conventional radioactivity measurement method for  Sr requires a complicated separation of
Sr requires a complicated separation of  Y and a time-consuming measurement. ICP-MS system has been applied to
Y and a time-consuming measurement. ICP-MS system has been applied to  Sr concentration survey of water, soil, and edible part of fish. We applied the ICP-MS system for the bones for the first time. In this study, reference bone (JSAC 0785 fish bone) was used as measurement samples. Sample preparation of the bone was performed using a microwave digestion instrument. After sample preparation,
Sr concentration survey of water, soil, and edible part of fish. We applied the ICP-MS system for the bones for the first time. In this study, reference bone (JSAC 0785 fish bone) was used as measurement samples. Sample preparation of the bone was performed using a microwave digestion instrument. After sample preparation,  Sr was determined using ICP-MS system with cascade separation steps based on on-line column separation and oxygen reaction. Strontium-90 in the bones was successfully separated from Ca, Ba, Y, Zr, Fe, Se, and Ge, which interfered in ICP-MS measurement, in the separation steps.
Sr was determined using ICP-MS system with cascade separation steps based on on-line column separation and oxygen reaction. Strontium-90 in the bones was successfully separated from Ca, Ba, Y, Zr, Fe, Se, and Ge, which interfered in ICP-MS measurement, in the separation steps.
Tachi, Yukio; Sato, Tomofumi*; Akagi, Yosuke*; Kawamura, Makoto*; Nakane, Hideji*; Terashima, Motoki; Fujiwara, Kenso; Iijima, Kazuki
Science of the Total Environment, 724, p.138098_1 - 138098_11, 2020/07
Times Cited Count:18 Percentile:57.06(Environmental Sciences)To understand and predict radiocesium transport behaviors in the environment, highly contaminated sediments from Ukedo and Odaka rivers around the Fukushima Daiichi Nuclear Power Plant were investigated systematically focusing on key factors controlling radiocesium sorption and fixation, including particle size, clay mineralogy and organic matter.
Terashima, Motoki; Endo, Takashi*; Miyakawa, Kazuya
Journal of Nuclear Science and Technology, 57(4), p.380 - 387, 2020/04
Times Cited Count:2 Percentile:17.25(Nuclear Science & Technology)Saito, Takumi*; Aoyagi, Noboru; Terashima, Motoki
Journal of Nuclear Science and Technology, 54(4), p.444 - 451, 2017/04
Times Cited Count:7 Percentile:51.25(Nuclear Science & Technology)Humic substances (HSs) are ubiquitous in various environments including deep underground and play an important role in the speciation and mobility of radionuclides. The binding of Eu , a chemical homologue of trivalent actinide ions, to HSs isolated from sedimentary groundwater at -250 m below the surface was studied by time-resolved laser fluorescence spectroscopy combined with parallel factor analysis (PARAFAC) as a function of pH and salt concentration. PARAFAC modeling reveals the presence of multiple factors that corresponds to different Eu
, a chemical homologue of trivalent actinide ions, to HSs isolated from sedimentary groundwater at -250 m below the surface was studied by time-resolved laser fluorescence spectroscopy combined with parallel factor analysis (PARAFAC) as a function of pH and salt concentration. PARAFAC modeling reveals the presence of multiple factors that corresponds to different Eu species. These factors resemble those observed for Eu
species. These factors resemble those observed for Eu binding to HSs from surface environments; however, detailed comparison shows that there are some particularities in Eu
binding to HSs from surface environments; however, detailed comparison shows that there are some particularities in Eu binding to the deep groundwater HSs. The distribution coefficients (
binding to the deep groundwater HSs. The distribution coefficients ( ) of Eu
) of Eu binding to the HSs calculated from the PARAFAC modeling exhibits a rather strong salt effect. At 0.01 M NaClO
binding to the HSs calculated from the PARAFAC modeling exhibits a rather strong salt effect. At 0.01 M NaClO the
the  values are relatively large and comparable to those to the surface HSs; they are decreaed at 0.1 M NaClO
values are relatively large and comparable to those to the surface HSs; they are decreaed at 0.1 M NaClO by more than an order of the magnitude. The
by more than an order of the magnitude. The  values are larger for humic acid fraction of the deep underground HSs than fulvic acid over the entire range of pH and salt concentration investigated in this study.
values are larger for humic acid fraction of the deep underground HSs than fulvic acid over the entire range of pH and salt concentration investigated in this study.
Saito, Takumi; Terashima, Motoki; Aoyagi, Noboru; Nagao, Seiya*; Fujitake, Nobuhide*; Onuki, Toshihiko
Environmental Science; Processes & Impacts, 17(8), p.1386 - 1395, 2015/08
Times Cited Count:9 Percentile:29.79(Chemistry, Analytical)The deep groundwater HSs were different from surface HSs, having high aliphaticities, sulfur contents, and small molecular sizes. The amounts of their acidic functional groups were comparable to or slightly larger than those of surface HSs; however, the magnitude of Cu binding to the deep groundwater HSs was smaller. The NICA-Donnan model attributed this to the binding of Cu
binding to the deep groundwater HSs was smaller. The NICA-Donnan model attributed this to the binding of Cu to chemically homogeneous carboxylic-type sites via mono-dentate coordination at relatively low pH. The binding mode tended to shift to multi-dentate coordination with carboxylic-type and probably more heterogeneous alcoholic hydroxide-type groups at higher pH. This study shows the particularity of the deep groundwater HSs in terms of their physicochemical and ion-binding properties, compared with surface HSs.
to chemically homogeneous carboxylic-type sites via mono-dentate coordination at relatively low pH. The binding mode tended to shift to multi-dentate coordination with carboxylic-type and probably more heterogeneous alcoholic hydroxide-type groups at higher pH. This study shows the particularity of the deep groundwater HSs in terms of their physicochemical and ion-binding properties, compared with surface HSs.
Terashima, Motoki; Nagao, Seiya*; Iwatsuki, Teruki; Fujitake, Nobuhide*; Seida, Yoshimi*; Iijima, Kazuki; Yoshikawa, Hideki
Journal of Nuclear Science and Technology, 49(8), p.804 - 815, 2012/08
Times Cited Count:13 Percentile:63.74(Nuclear Science & Technology)Seida, Yoshimi; Terashima, Motoki; Tachi, Yukio; Iijima, Kazuki; Nakazawa, Toshiyuki*; Yamada, Norikazu*; Yui, Mikazu
Radiochimica Acta, 98(9-11), p.703 - 709, 2010/11
Times Cited Count:3 Percentile:23.02(Chemistry, Inorganic & Nuclear)Diffusion and sorption behaviors of Eu in sedimentary rock were investigated in the presence of humic substance in the present study. Sedimentary rock obtained from Horonobe URL test site in Hokkaido, Japan, (accessible pore diameter is around several to several ten nanometer) was used. The Diffusion behaviors of Eu were examined based on reservoir depletion method coupled with analysis of inner distribution of the radioactive elements in the rock. Time sequence of concentration of each radioactive element as well as the humic substance in the reservoir was obtained as a function of concentration and molecular size of humic substance. Coexistence of humic substance reduced the depletion of Eu in the reservoir, indicating complexation between the radioactive elements and the humic substance. On the contrary, obvious decrease of humic substance in the reservoir was not observed in the system. This observation suggests that the radioactive elements became hard to diffuse into the sedimentary rock due to an increase of their size through complexation with the humic substance. The sorption of Eu was reduced with increase of the humic substance although the sorption of the humic substance was not influenced by the existence of Th or Eu. The diffusion and sorption of Eu were found to be reduced in the presence of humic substance.