Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O )
) -(SiO
-(SiO )
) measured by aerodynamic levitation
measured by aerodynamic levitationKondo, Toshiki; Toda, Taro*; Takeuchi, Junichi*; Kikuchi, Shin; Kargl, F.*; Muta, Hiroaki*; Oishi, Yuji*
High Temperatures-High Pressures, 52(3-4), p.307 - 321, 2023/06
Times Cited Count:0 Percentile:0.02(Thermodynamics)In order to establish an evaluation method/numerical simulation for nuclear reactor safety under severe accidental conditions, it is necessary to obtain the physical properties, especially fluidity of the relevant molten materials at very high temperatures. In this study, thermophysical properties such as density and viscosity were obtained for (Fe O
O )
) -(SiO
-(SiO )
) , which is a representative composition in the early stage of severe accident. (Fe
, which is a representative composition in the early stage of severe accident. (Fe O
O )
) -(SiO
-(SiO )
) is produced by the contact between the molten oxide of steel, which is the main component of the reactor, and SiO
is produced by the contact between the molten oxide of steel, which is the main component of the reactor, and SiO , which is the main component of concrete. As a result, the physical properties of the (Fe
, which is the main component of concrete. As a result, the physical properties of the (Fe O
O )
) -(SiO
-(SiO )
) mixture were almost the same as those of Fe
mixture were almost the same as those of Fe O
O obtained in previous studies, and it could be concluded that a small amount of SiO
obtained in previous studies, and it could be concluded that a small amount of SiO (about 5 mol.%) did not significantly affect the fluidity of Fe
(about 5 mol.%) did not significantly affect the fluidity of Fe O
O .
.
 , (FeO
, (FeO )
)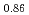 -(ZrO
-(ZrO )
)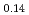 and (FeO
and (FeO )
)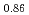 -(UO
-(UO )
)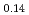
Kondo, Toshiki; Toda, Taro*; Takeuchi, Junichi*; Kargl, F.*; Kikuchi, Shin; Muta, Hiroaki*; Oishi, Yuji*
Journal of Nuclear Science and Technology, 59(9), p.1139 - 1148, 2022/09
Times Cited Count:1 Percentile:31.61(Nuclear Science & Technology)no abstracts in English
 hydrogen partitioning control
hydrogen partitioning controlToda, Hiroyuki*; Yamaguchi, Masatake; Matsuda, Kenji*; Shimizu, Kazuyuki*; Hirayama, Kyosuke*; Su, H.*; Fujihara, Hiro*; Ebihara, Kenichi; Itakura, Mitsuhiro; Tsuru, Tomohito; et al.
Tetsu To Hagane, 105(2), p.240 - 253, 2019/02
Times Cited Count:0 Percentile:0(Metallurgy & Metallurgical Engineering)no abstracts in English
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.649 - 651, 2009/08
Times Cited Count:8 Percentile:19.4(Electrochemistry)The distribution coefficients of Am and Ce were measured in the eutectic LiCl-KCl/liquid Ga system at 773K. By using ZrCl as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO
as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO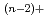 , the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
, the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
Moriyama, Hirotake*; Moritani, Kimikazu*; Toda, Taro*; Hayashi, Hirokazu
Radiochimica Acta, 97(4-5), p.233 - 236, 2009/05
Times Cited Count:1 Percentile:10.22(Chemistry, Inorganic & Nuclear)The distribution coefficients of Am, Ce, and Eu between the salt and metal phases were measured at 1073 K in a reductive extraction system of equimolar NaCl-KCl melt and liquid Ga, which were expected to be higher from a thermodynamic point of view. By changing some solute concentrations, it was observed that the distribution coefficients were much dependent on the oxide ion concentration in the system, possibly due to the formation of such compounds as AmO and CeO
and CeO . Also, in some runs, the mass balance of Am was affected possibly by the formation and precipitation of its oxychloride and/or oxide. By taking the observations into consideration, the separation factor between Am and Ce was obtained to be about 30 as a lower limit in the present system.
. Also, in some runs, the mass balance of Am was affected possibly by the formation and precipitation of its oxychloride and/or oxide. By taking the observations into consideration, the separation factor between Am and Ce was obtained to be about 30 as a lower limit in the present system.
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Journal of Nuclear Science and Technology, 46(1), p.18 - 25, 2009/01
Times Cited Count:41 Percentile:92.56(Nuclear Science & Technology)The excess thermodynamic quantities of lanthanides and actinides in molten salts and liquid metals were studied for reductive extraction of minor actinides. The excess enthalpies and entropies of those elements in the molten chloride phase were found to be correlated with the ionic radii of metal ions possibly due to complex formation. In the liquid metal phase, on the other hand, the excess enthalpies were explained with Miedema's atomistic model and the excess entropies were explained with the vibrational entropy due to alloy formation. Using these correlations and models, some missing values of the excess thermodynamic quantities were evaluated and the separation factors of minor actinides from lanthanides were calculated in different reductive extraction systems. The higher separation factors were obtained in the system using aluminum or gallium than in the system using bismuth or cadmium as the liquid metal phase.
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Proceedings of 2008 Joint Symposium on Molten Salts (USB Flash Drive), p.933 - 938, 2008/10
The distribution coefficients of Am and Ce were measured in the LiCl-KCl/Ga system at 773 K. By using ZrCl as the oxide ion scavenger in order to avoid the formation of such oxychlorides as CeO
as the oxide ion scavenger in order to avoid the formation of such oxychlorides as CeO and AmO
and AmO , the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that solvent metals were ordered from the most selective to the less selective one as Al
, the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that solvent metals were ordered from the most selective to the less selective one as Al Ga
Ga Bi
Bi Cd.
Cd.
Suzuki, Ryosuke*; Tanabe, Kentaro*; kondo, koki*; Ono, Katsutoshi*; Toda, Shinichi; Kasagawa, Yusuke; Tamayama, Kiyoshi; Oketani, Kazuhiro*
JNC TY4400 2003-004, 214 Pages, 2003/08
Recently, it has been important to reuse discharged heat energy from present nuclear plants in the view of reduction of environmental burden and improvement of heat efficiency for plant. For practical use in future of sodium cooled FBRs, which are typical high temperature system, this issue must be given priority. The thermal electric conversion system has been applied to the limited uses such as space or military, however, that results show good merits for reliability, maintenance free, and so on. Recently, this technology has been reconsidered in the view of saving energy in general industry. In this study, we made an investigation for applicability of the thermal electric conversion system to sodium cooled FBR as a heat recovery techbnology. Exactly, We have carried out the fundamental research and development for thermoelectric materials and elements, development of modules, and sodium tests with those modules, and then, we acquired the fundamental knowledge to estimate the efficiencies of thermal electric conversion system or modules for a sodium cooled FBR.
Tanaka, Takuro*; Saito, Takumi*; Toda, Kanako*; Fujiwara, Kenso; Terashima, Motoki; Nakanishi, Takahiro; Kobayashi, Natsuko*; Tanoi, Keitaro*
no journal, ,
Cs-137 dispersed by the Fukushima Daiichi Nuclear Power Plant accident deposited in Fukushima area, that are not decontaminated in mountainous areas, may migrate through river water to animals and plants. Most of Cs-137 in river are fixed in clay minerals, but there are some Cs that can be easily desorbed, named as labile components. It has been suggested that labile components affect the bioavailability of Cs-137. In this study, the labile Cs-137 was sampled in situ from upstream to downstream in several rivers of Fukushima using a passive sampler called diffusive gradients in thin films (DGT). Sampling was conducted at a different season in addition to the previous studies.
 O
O )
) -(SiO
-(SiO )
) by aerodynamic levitation
by aerodynamic levitationKondo, Toshiki; Toda, Taro*; Takeuchi, Junichi*; Kikuchi, Shin; Kargl, F.*; Muta, Hiroaki*; Oishi, Yuji*
no journal, ,
 Cs in Fukushima rivers by diffusive gradients in thin films (DGT), 3; Relations between geochemical parameters
Cs in Fukushima rivers by diffusive gradients in thin films (DGT), 3; Relations between geochemical parametersTanaka, Takuro*; Saito, Takumi*; Toda, Kanako*; Fujiwara, Kenso; Terashima, Motoki; Nakanishi, Takahiro; Kobayashi, Natsuko*; Tanoi, Keitaro*; Kato, Hiroaki*
no journal, ,
 Cs dispersed by the Fukushima Daiichi Nuclear Power Plant accident deposited in Fukushima area, that are not decontaminated in mountainous areas, may migrate through river water to animals and plants. Most of
Cs dispersed by the Fukushima Daiichi Nuclear Power Plant accident deposited in Fukushima area, that are not decontaminated in mountainous areas, may migrate through river water to animals and plants. Most of  Cs in river are fixed in clay minerals, but there are some Cs that can be easily desorbed, named as labile components. It has been suggested that labile components affect the bioavailability of
Cs in river are fixed in clay minerals, but there are some Cs that can be easily desorbed, named as labile components. It has been suggested that labile components affect the bioavailability of  Cs. In this study, the labile
Cs. In this study, the labile  Cs was sampled in situ from upstream to downstream in several rivers of Fukushima using a passive sampler called diffusive gradients in thin films (DGT). The desorption behavior of the labile component and factors affecting it will be discussed by examining the relationship with particulate
Cs was sampled in situ from upstream to downstream in several rivers of Fukushima using a passive sampler called diffusive gradients in thin films (DGT). The desorption behavior of the labile component and factors affecting it will be discussed by examining the relationship with particulate  Cs and geochemical parameters in river water.
Cs and geochemical parameters in river water.