Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tomita, Ryohei; Tomita, Jumpei; Suzuki, Daisuke; Yasuda, Kenichiro; Miyamoto, Yutaka
Hosha Kagaku, (48), p.1 - 15, 2023/09
Secondary Ion Mass Spectrometry (SIMS) is the method to detect secondary ions produced by the sputtering of primary ions. SIMS is one of effective method to measure isotopic composition of particles containing nuclear material in environmental sample for safeguards. We are a group member of the International Atomic Energy Agency (IAEA)'s network of analytical laboratories and have developed analytical techniques using SIMS and other mass spectrometers for nuclear safeguards. We will introduce the principle of SIMS and analytical techniques developed by our group to measure isotopic composition of uranium particles which having a particle diameter of micron order in environmental sample for safeguards.
Miyamoto, Yutaka; Suzuki, Daisuke; Tomita, Ryohei; Tomita, Jumpei; Yasuda, Kenichiro
Isotope News, (786), p.22 - 25, 2023/04
no abstracts in English
Tomita, Ryohei; Tomita, Jumpei; Yomogida, Takumi; Suzuki, Daisuke; Yasuda, Kenichiro; Esaka, Fumitaka; Miyamoto, Yutaka
KEK Proceedings 2022-2, p.108 - 113, 2022/11
Automated Particle Measurement (APM) is the first measurement of environmental sample for safeguard purpose. APM tells us the number of particles in sample, their enrichment and their location. Precision and accuracy of APM is easily affected by particle condition. We have investigated how influential baking temperature in sample preparation are for uranium secondary ion quantity, uranium hydride generation and particle crystallinity. Our experimental results showed that baking temperature of 800 C reduced uranium secondary ion quantity to 33% compared with baking at 350
C reduced uranium secondary ion quantity to 33% compared with baking at 350 C. Uranium hydride generation ratio of the sample baked at 850
C. Uranium hydride generation ratio of the sample baked at 850 C was also 4 times higher than the sample baked at 350
C was also 4 times higher than the sample baked at 350 C. Baking at 850
C. Baking at 850 C raised only crystallinity of uranium particles. Baking sample at too high temperature caused less uranium secondary ion generation and much more uranium hydride generation. It made precision and accuracy of APM worse. In our experiment, baking at 350
C raised only crystallinity of uranium particles. Baking sample at too high temperature caused less uranium secondary ion generation and much more uranium hydride generation. It made precision and accuracy of APM worse. In our experiment, baking at 350 C is suitable for uranium particles in the safeguards sample.
C is suitable for uranium particles in the safeguards sample.
Tomita, Jumpei; Tomita, Ryohei; Suzuki, Daisuke; Yasuda, Kenichiro; Miyamoto, Yutaka
KEK Proceedings 2022-2, p.154 - 158, 2022/11
Precise determination of minor U isotopes ( U and
U and 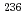 U) of particles from the safeguard environmental samples is powerful method for detecting the undeclared nuclear activities. In this study, preparation method of U particle was examined to utilize for the minor U isotope determination. The porous silica particles were used as the particle matrix and lutetium was mixed to the impregnation solution as U impregnation indicator for the particle picking. The result of the Scanning Electron Microscope indicated that the contacting the solution with Si particles overnight gently could produce the impregnated particles effectively rather than the mixing them with PFA stick.
U) of particles from the safeguard environmental samples is powerful method for detecting the undeclared nuclear activities. In this study, preparation method of U particle was examined to utilize for the minor U isotope determination. The porous silica particles were used as the particle matrix and lutetium was mixed to the impregnation solution as U impregnation indicator for the particle picking. The result of the Scanning Electron Microscope indicated that the contacting the solution with Si particles overnight gently could produce the impregnated particles effectively rather than the mixing them with PFA stick.
Tomita, Ryohei; Tomita, Jumpei; Yomogida, Takumi; Suzuki, Daisuke; Yasuda, Kenichiro; Esaka, Fumitaka; Miyamoto, Yutaka
KEK Proceedings 2021-2, p.146 - 150, 2021/12
no abstracts in English
Miyabe, Masabumi; Satou, Yukihiko; Wakaida, Ikuo; Terabayashi, Ryohei*; Sonnenschein, V.*; Tomita, Hideki*; Zhao, Y.*; Sakamoto, Tetsuo*
Journal of Physics B; Atomic, Molecular and Optical Physics, 54(14), p.145003_1 - 145003_8, 2021/07
Times Cited Count:0 Percentile:0.01(Optics)Two-color two-step photoionization optogalvanic spectroscopy was performed using high-repetition-rate titanium sapphire lasers and a uranium hollow cathode lamp to find the two-step resonance ionization schemes of uranium. Many ionization transitions were observed by exciting uranium atoms in a ground state into five, even parity, excited levels with the first-step laser and by scanning the second-step laser wavelengths. By blocking the first-step laser, single-color, two-photon ionization transitions were also identified. From these results, we have found more than 50 odd-parity autoionizing levels of uranium in the energy, ranging from the ionization potential (49958.4 cm ) to 51150 cm
) to 51150 cm . The determined energy levels are within 1 cm
. The determined energy levels are within 1 cm of previously reported values.
of previously reported values.
Suzuki, Daisuke; Tomita, Ryohei; Tomita, Jumpei; Esaka, Fumitaka; Yasuda, Kenichiro; Miyamoto, Yutaka
Journal of Radioanalytical and Nuclear Chemistry, 328(1), p.103 - 111, 2021/04
Times Cited Count:3 Percentile:45.99(Chemistry, Analytical)An analytical technique was developed to determine the age of uranium particles for safeguards. After the chemical separation of uranium and thorium, the 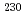 Th/
Th/ U ratio was measured using single-collector inductively coupled plasma mass spectrometry and a
U ratio was measured using single-collector inductively coupled plasma mass spectrometry and a  U-based reference material comprising a certain amount of
U-based reference material comprising a certain amount of 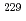 Th as a progeny nuclide of
Th as a progeny nuclide of  U. The results allowed us to determine the purification age of two certified materials, i.e., U-850 and U-100, which was in good agreement with the reference purification age (61 y). Moreover, the age of a single U-850 particle was determined with a difference of -28 to 2 years from the reference date.
U. The results allowed us to determine the purification age of two certified materials, i.e., U-850 and U-100, which was in good agreement with the reference purification age (61 y). Moreover, the age of a single U-850 particle was determined with a difference of -28 to 2 years from the reference date.
Tomita, Ryohei*; Matsunaka, Tetsuya*; Honda, Maki*; Satou, Yukihiko; Matsumura, Masumi*; Takahashi, Tsutomu*; Sakaguchi, Aya*; Matsuzaki, Hiroyuki*; Sasa, Kimikazu*; Sueki, Keisuke*
no journal, ,
no abstracts in English
Esaka, Fumitaka; Yomogida, Takumi; Tomita, Ryohei; Miyamoto, Yutaka
no journal, ,
Chemical state analysis is important to elucidate the origin of particles and its effect on the environment. However, the analysis is difficult because the amounts of elements in individual particles are considerably small. In the present study, electron backscatter diffraction (EBSD) is applied to the chemical state analysis of individual uranium particles. As a result, uranium particles with diameters of 1 m were able to be measured and clear electron backscatter patterns were observed. In addition, the patterns of UO
m were able to be measured and clear electron backscatter patterns were observed. In addition, the patterns of UO particles were distinguished from those of U
particles were distinguished from those of U O
O particles.
particles.
Tomita, Ryohei; Esaka, Fumitaka; Miyamoto, Yutaka
no journal, ,
In the previous study, we developed a combination method of particle manipulation and secondary ion mass spectrometry (SIMS) for removing the interferences from other elements in other particles. Since the particles were selected randomly and manipulated, it caused the problem not to cover the range of  U/
U/ U isotope ratio in the sample. In this study, we improved the procedure of analyzing uranium isotope ratios in individual particles by SIMS to cover the range of
U isotope ratio in the sample. In this study, we improved the procedure of analyzing uranium isotope ratios in individual particles by SIMS to cover the range of  U/
U/ U in the sample.
U in the sample.
Tomita, Ryohei; Esaka, Fumitaka; Miyamoto, Yutaka
no journal, ,
In the previous study, we developed a combination method of individual uranium particle manipulation in scanning electron microscope (SEM) and secondary ion mass spectrometry (SIMS) for removing the interferences from other elements in other particles. Since the particles were selected randomly and manipulated, it caused the problem not to cover the range of  U/
U/ U isotope ratio in the sample. In this study, we introduced pre-screening measurement into the procedure of analyzing uranium isotope ratios in individual particles by SIMS to cover the range of
U isotope ratio in the sample. In this study, we introduced pre-screening measurement into the procedure of analyzing uranium isotope ratios in individual particles by SIMS to cover the range of  U/
U/ U in the sample.
U in the sample.
Tomita, Ryohei; Esaka, Fumitaka; Yasuda, Kenichiro; Suzuki, Daisuke; Miyamoto, Yutaka
no journal, ,
The research group for safeguards analytical chemistry which belong to Japan Atomic Energy Agency (JAEA) introduced new Large Geometry Secondary Ion Mass Spectrometry (LG-SIMS) instrument (1300-HR , CAMECA) to update our capability. LG-SIMS instrument has higher mass resolving power (MRP) than small geometry SIMS (SG-SIMS) instrument we had used. We hope molecular interferences (e.g. Pb, Al, Fe) are removed by LG-SIMS instrument when we analyze uranium bearing particles in environmental sample. In order to obtain the bes LG-SIMS condition for uranium particle analysis, we investigated a relationship between MRP, secondary ion's intensity and peak shape of uranium. One of the most famous molecular interferences of uranium SIMS analysis is PbAl
, CAMECA) to update our capability. LG-SIMS instrument has higher mass resolving power (MRP) than small geometry SIMS (SG-SIMS) instrument we had used. We hope molecular interferences (e.g. Pb, Al, Fe) are removed by LG-SIMS instrument when we analyze uranium bearing particles in environmental sample. In order to obtain the bes LG-SIMS condition for uranium particle analysis, we investigated a relationship between MRP, secondary ion's intensity and peak shape of uranium. One of the most famous molecular interferences of uranium SIMS analysis is PbAl (mass number: 234, 235). If we attempt to remove molecular interferences of the PbAl
(mass number: 234, 235). If we attempt to remove molecular interferences of the PbAl from uranium mass regions, MRP value of 2741 is required. The MRP of LG-SIMS instrument is defined by entrance and exit slit. We need to keep balance between MRP, secondary ion's intensity and flat-top peak rate. We compared some combination of entrance and exit slit to get better balance. We found the best combination of entrance and exit slit is sized 200
from uranium mass regions, MRP value of 2741 is required. The MRP of LG-SIMS instrument is defined by entrance and exit slit. We need to keep balance between MRP, secondary ion's intensity and flat-top peak rate. We compared some combination of entrance and exit slit to get better balance. We found the best combination of entrance and exit slit is sized 200  m and 500
m and 500  m, respectively. LG-SIMS can almost remove PbAl
m, respectively. LG-SIMS can almost remove PbAl from uranium secondary ions in this condition. This research is part of investigation into safeguards analysis techniques commissioned by nuclear regulatory agency.
from uranium secondary ions in this condition. This research is part of investigation into safeguards analysis techniques commissioned by nuclear regulatory agency.
Tomita, Jumpei; Tomita, Ryohei; Suzuki, Daisuke; Yasuda, Kenichiro; Miyamoto, Yutaka
no journal, ,
no abstracts in English
Tomita, Ryohei; Esaka, Fumitaka; Yomogida, Takumi; Miyamoto, Yutaka
no journal, ,
Large Geometry Secondary Ion Mass Spectrometry (LG-SIMS) is one of the strongest tools for analyzing isotope ratios of micron sized uranium particle. LG-SIMS has high spatial resolution of less than 1  m with microprobe mode. However, this capability is bit less under automated particle measurement (APM) condition. If two or more particles are located in a quite narrow area, APM may detect the cluster as one particle. This particle mixing effect shows analytical results including false isotope ratios. In order to investigate how often particle mixing happens and how to solve this problem, we implemented APM to mixed uranium particle standard (U010, U100, U350 and U850) and try to apply particle manipulation with APM. In our experiment, each area (350
m with microprobe mode. However, this capability is bit less under automated particle measurement (APM) condition. If two or more particles are located in a quite narrow area, APM may detect the cluster as one particle. This particle mixing effect shows analytical results including false isotope ratios. In order to investigate how often particle mixing happens and how to solve this problem, we implemented APM to mixed uranium particle standard (U010, U100, U350 and U850) and try to apply particle manipulation with APM. In our experiment, each area (350 350
350  m
m ) was scanned with an O
) was scanned with an O primary beam with a current of 1.5 nA for 9 sec. Then, secondary ion images were recorded for circular area with a radius of 8500
primary beam with a current of 1.5 nA for 9 sec. Then, secondary ion images were recorded for circular area with a radius of 8500  m on the center of a sample planchet. The APM detected 5976 particles, and 1943 particles (32%) in them showed false isotope ratios. In addition to particle mixing, U850 cluster was shifted down to around 75% enrichment. The sample showed too high hydride rate, so that
m on the center of a sample planchet. The APM detected 5976 particles, and 1943 particles (32%) in them showed false isotope ratios. In addition to particle mixing, U850 cluster was shifted down to around 75% enrichment. The sample showed too high hydride rate, so that  UH interfered
UH interfered 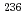 U. This interference caused false
U. This interference caused false 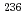 U abundance and lead to false
U abundance and lead to false  U abundance. To solve these problems, 50
U abundance. To solve these problems, 50 80 particles were manipulated randomly from another mixed standards planchet and analyzed them by APM.
80 particles were manipulated randomly from another mixed standards planchet and analyzed them by APM.
Tomita, Ryohei; Tomita, Jumpei; Yomogida, Takumi; Suzuki, Daisuke; Yasuda, Kenichiro; Esaka, Fumitaka; Miyamoto, Yutaka
no journal, ,
Secondary ion mass spectrometry (SIMS) analysis of uranium particles for safeguards purpose consists of Automated Particle Measurement (APM) and Microprobe analysis. APM for safeguards sample includes 2400 measurements, each field is analyzed for short time. So, if a sample have particles which generate too much uranium hydride formation on their surface, the APM result, especially 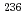 U abundance, is affected by uranium hydride formation. It causes inaccurate APM result. To investigate what percentage of the entire particle the particle surface causing ratio change account for, total evaporation measurement was implemented for standard uranium particle generating much uranium hydride formation on their surface and uranium isotopic ratio change during the total evaporation measurement was observed. Total evaporation experiment indicated that the number of secondary ions originated from particle surface accounted for 3.1% of all of number of ions sputtered from the entire particle. Based on the total evaporation result, APM conditions, primary beam intensity, measurement time and raster size, combined with the method manipulating particles under scanning electron microscope were optimized to reduce the hydride effect for APM result.
U abundance, is affected by uranium hydride formation. It causes inaccurate APM result. To investigate what percentage of the entire particle the particle surface causing ratio change account for, total evaporation measurement was implemented for standard uranium particle generating much uranium hydride formation on their surface and uranium isotopic ratio change during the total evaporation measurement was observed. Total evaporation experiment indicated that the number of secondary ions originated from particle surface accounted for 3.1% of all of number of ions sputtered from the entire particle. Based on the total evaporation result, APM conditions, primary beam intensity, measurement time and raster size, combined with the method manipulating particles under scanning electron microscope were optimized to reduce the hydride effect for APM result.
Tomita, Jumpei; Tomita, Ryohei; Suzuki, Daisuke; Yasuda, Kenichiro; Miyamoto, Yutaka
no journal, ,
Formation of polyatomic interferences made of an atom of heavy element and atoms in plasma such as argon and oxygen is known to create problems for their measurements using ICP-MS. In this study, quantitative assessment of polyatomic interferences for the measurement of U and Pu isotope ratios at ultra-trace level using MC-ICP-MS was conducted. For U isotopes, significant polyatomic interferences caused by 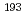 Ir
Ir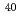 Ar,
Ar, 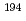 Pt
Pt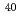 Ar and
Ar and 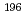 Pt
Pt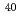 Ar were observed at the mass of 233, 234 and 236, respectively. When 1 ppb of natural uranium solution (IRMM184) containing 0.4 ppb of Pt was measured,
Ar were observed at the mass of 233, 234 and 236, respectively. When 1 ppb of natural uranium solution (IRMM184) containing 0.4 ppb of Pt was measured,  U/
U/ U isotope ratio was roughly estimated to be two-fold higher than certified value due to the interference. For Pu isotopes, small interference from Pb (
U isotope ratio was roughly estimated to be two-fold higher than certified value due to the interference. For Pu isotopes, small interference from Pb (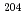 Pb
Pb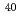 Ar) was observed at the mass of 244 while other obvious interferences were not found.
Ar) was observed at the mass of 244 while other obvious interferences were not found.
Tomita, Jumpei; Tomita, Ryohei; Suzuki, Daisuke; Yasuda, Kenichiro; Miyamoto, Yutaka
no journal, ,
Isotopic ratios of uranium particle provide us with the information on the nuclear activities such as enrichment and reprocessing. Precise determination of U isotopic ratios is difficult due to the low intensity of  U measured by Faraday cup when pico-gram quantities of uranium was measured by MC-ICP-MS. In this study, the sensitive measurement of the 1-20 pg of uranium was examined. The solution was prepared by only 0.2 mL, which was one-tenth compared to the conventional method, to increase U concentration. Data acquisition was started from the beginning of the solution uptake and continued until all solution was exhausted. The isotopic ratios of uranium were calculated from the total counts of each isotope excepting the portion affected by air mixing at the beginning and end of sample introduction. Uranium isotopic ratios of CRM U015 and IRMM184 determined by this method examined in this study were agreed with the certified values within the uncertainties (2-sigma). The uncertainties obtained by this method were smaller than those by the conventional method.
U measured by Faraday cup when pico-gram quantities of uranium was measured by MC-ICP-MS. In this study, the sensitive measurement of the 1-20 pg of uranium was examined. The solution was prepared by only 0.2 mL, which was one-tenth compared to the conventional method, to increase U concentration. Data acquisition was started from the beginning of the solution uptake and continued until all solution was exhausted. The isotopic ratios of uranium were calculated from the total counts of each isotope excepting the portion affected by air mixing at the beginning and end of sample introduction. Uranium isotopic ratios of CRM U015 and IRMM184 determined by this method examined in this study were agreed with the certified values within the uncertainties (2-sigma). The uncertainties obtained by this method were smaller than those by the conventional method.
Tomita, Ryohei; Tomita, Jumpei; Suzuki, Daisuke; Yasuda, Kenichiro; Esaka, Fumitaka; Miyamoto, Yutaka
no journal, ,
It is necessary to correctly calibrate the mass bias effect of uranium isotopes using uranium standard particles in the secondary ion mass spectrometry (SIMS) analysis. The preparation of uranium standard particles is mainly carried out by drying aerosols generated from uranium standard solutions in unique equipment and facility. This is the reason why only few types of commercial uranium standard particles are available. In this study, our purpose is to propose easier way to prepare uranium standard particle by immersing porous silicon particle in the uranium standard solution. Quality of this handmade uranium standard particles were evaluated by analyzing isotopic ratios using SIMS. The uranium isotopic standard solution ( U/
U/ U=0.694,
U=0.694,  U/
U/ U=0.922) of 2.21 ppm was concentrated to 4.48
U=0.922) of 2.21 ppm was concentrated to 4.48 10
10 ppm, and mixed with porous silicon particle. Uranium isotopic ratios of handmade particles collected on a glassy carbon planchet were analyzed using LG-SIMS (IMS-1300HR
ppm, and mixed with porous silicon particle. Uranium isotopic ratios of handmade particles collected on a glassy carbon planchet were analyzed using LG-SIMS (IMS-1300HR , CAMECA). Analytical results of
, CAMECA). Analytical results of  U/
U/ U and
U and  U/
U/ U agreed with the certified value of standard solution within the standard deviation (1
U agreed with the certified value of standard solution within the standard deviation (1 ). This new particle preparation is effective to create standard particles without uranium aerosol, and the particles made by this method showed same isotopic ratios as standard solution in which porous silicon particles was immerged.
). This new particle preparation is effective to create standard particles without uranium aerosol, and the particles made by this method showed same isotopic ratios as standard solution in which porous silicon particles was immerged.
Tomita, Ryohei; Tomita, Jumpei; Suzuki, Daisuke; Yasuda, Kenichiro; Miyamoto, Yutaka
no journal, ,
We are applying a secondary ion mass spectrometer (SIMS) to measure the isotopic composition of micron-sized nuclear particles in environmental samples for safeguards purposes. International Atomic Energy Agency (IAEA) collects swipe samples taken from the walls and floors of nuclear facilities through on-site inspections, and analyzes uranium isotopic composition of these samples for confirming the absence of undeclared nuclear activity. As a member of IAEA network analytical laboratories (NWALs), our research group has not only reported the analytical results of isotopic composition of U and Pu in the inspection samples to IAEA, but also has been developed analytical techniques to precisely and accurately measure the isotopic composition of nuclear materials on the IAEA swipe samples. Our analytical activity at Clean Laboratory for Environmental Analysis and Research (CLEAR) in JAEA, and analytical techniques using SIMS are introduced.
Yasuda, Kenichiro; Suzuki, Daisuke; Tomita, Jumpei; Tomita, Ryohei; Miyamoto, Yutaka
no journal, ,
The safeguards environmental sample analysis by the IAEA requires the development of efficient methods for measuring isotope ratios of ultra-trace amounts of plutonium and uranium particles. We have applied fission track and alpha track techniques to identify of discrimination between plutonium and uranium particles and have successfully measured isotope ratios of the particles using a continuous heating method with a thermal ionization mass spectrometer (TIMS). This method made it possible to find particles containing plutonium and uranium and measure them simultaneously by the TIMS without a chemical separation.