Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
JPS Conference Proceedings (Internet), 8, p.036004_1 - 036004_6, 2015/09
We aim to build a high-resolution neutron time-of-flight diffractometer for biomacromolecules at the Materials and Life Science Experimental Facility (MLF) at the Japan Proton Accelerator Research Complex (J-PARC) that allows the collection of neutron diffraction data from crystals with unit cells of  250
250  ;. Considering both the flux and pulse width necessary to realize data collection covering a minimum d-spacing of 2.0
;. Considering both the flux and pulse width necessary to realize data collection covering a minimum d-spacing of 2.0  ; and with a unit cell constant of
; and with a unit cell constant of  250
250  ; we chose a decoupled moderator (DM) as the appropriate source for this high-resolution diffractometer. We considered a simple instrumentation model that includes a moderator, neutron guide, sample size, and neutron detector; we then investigated its spot separation performance and estimated the instrumental parameters for the design of a new diffractometer for protein crystals with large unit cells at J-PARC/MLF. It is preferable to extend the total flight path to resolve Bragg reflections for protein crystals with large unit cells as the scattering angle increases. Meanwhile, to ensure resolvable detection capacity at the middle scattering angle region (2
; we chose a decoupled moderator (DM) as the appropriate source for this high-resolution diffractometer. We considered a simple instrumentation model that includes a moderator, neutron guide, sample size, and neutron detector; we then investigated its spot separation performance and estimated the instrumental parameters for the design of a new diffractometer for protein crystals with large unit cells at J-PARC/MLF. It is preferable to extend the total flight path to resolve Bragg reflections for protein crystals with large unit cells as the scattering angle increases. Meanwhile, to ensure resolvable detection capacity at the middle scattering angle region (2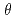
 90
90 ), it is necessary to restrict the angular divergence. In the case of
), it is necessary to restrict the angular divergence. In the case of 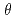

 0.2
0.2 , scattering angles from around 2
, scattering angles from around 2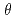
 90
90 to higher backscattering angles are more efficient for protein crystals with large unit cells (
to higher backscattering angles are more efficient for protein crystals with large unit cells ( 250
250  ) with a resolution of 2.0
) with a resolution of 2.0  .
.
Unno, Masayoshi*; Ishikawa, Kumiko*; Kusaka, Katsuhiro*; Tamada, Taro; Hagiwara, Yoshinori*; Sugishima, Masakazu*; Wada, Kei*; Yamada, Taro*; Tomoyori, Katsuaki; Hosoya, Takaaki*; et al.
Journal of the American Chemical Society, 137(16), p.5452 - 5460, 2015/04
Times Cited Count:29 Percentile:62.63(Chemistry, Multidisciplinary)Phycocyanobilin, a light-harvesting and photoreceptor pigment in higher plants, algae, and cyanobacteria, is synthesized from biliverdin IX (BV) by phycocyanobilin:ferredoxin oxidoreductase (PcyA) via two steps of two-proton-coupled two-electron reduction. We determined the neutron structure of PcyA from cyanobacteria complexed with BV, revealing the exact location of the hydrogen atoms involved in catalysis. Notably, approximately half of the BV bound to PcyA was BVH
(BV) by phycocyanobilin:ferredoxin oxidoreductase (PcyA) via two steps of two-proton-coupled two-electron reduction. We determined the neutron structure of PcyA from cyanobacteria complexed with BV, revealing the exact location of the hydrogen atoms involved in catalysis. Notably, approximately half of the BV bound to PcyA was BVH , a state in which all four pyrrole nitrogen atoms were protonated. The protonation states of BV complemented the protonation of adjacent Asp105. The "axial "water molecule that interacts with the neutral pyrrole nitrogen of the A-ring was identified. His88 N
, a state in which all four pyrrole nitrogen atoms were protonated. The protonation states of BV complemented the protonation of adjacent Asp105. The "axial "water molecule that interacts with the neutral pyrrole nitrogen of the A-ring was identified. His88 N was protonated to form a hydrogen bond with the lactam O atom of the BV A-ring. His88 and His74 were linked by hydrogen bonds via H
was protonated to form a hydrogen bond with the lactam O atom of the BV A-ring. His88 and His74 were linked by hydrogen bonds via H O
O . These results imply that Asp105, His88, and the axial water molecule contribute to proton transfer during PcyA catalysis.
. These results imply that Asp105, His88, and the axial water molecule contribute to proton transfer during PcyA catalysis.
Tomoyori, Katsuaki; Hirano, Yu; Kurihara, Kazuo; Tamada, Taro
Journal of Physics; Conference Series, 664, p.072049_1 - 072049_7, 2015/00
Times Cited Count:5 Percentile:84.12(Physics, Nuclear)In neutron protein crystallography, it should be also emphasized that the weak Bragg reflections due to the large unit cells may be buried beneath the strong background caused by the incoherent scattering of hydrogen atoms. Therefore, the background estimation from the source is more reliable to improve the accuracy of Bragg integral intensity. We propose the adoption of Statistics-sensitive Nonlinear Iterative Peak-clipping (SNIP) algorithm for background estimation, which can eliminate the background from the source spectrum as well as statistically enhance low peaks. In addition, it was recently reported that the Landau and Vavilov distributions, which are used to describe the energy loss of charged particles traversing a thin absorber were found to be in excellent agreement with the observed TOF profile. I show that it may be beneficial to establish a profile-fitting method with the combined use of the Landau/Vavilov functions to faithfully reproduce the TOF peak shape and a SNIP background evaluation algorithm to eliminate statistical fluctuations.
Tomoyori, Katsuaki; Kusaka, Katsuhiro*; Yamada, Taro*; Tamada, Taro
Journal of Structural and Functional Genomics, 15(3), p.131 - 135, 2014/09
We plan to design a high-resolution biomacromolecule neutron time-of-flight diffractometer, which allows us to collect data from crystals with unit cells above 250  in the Materials and Life Science Experimental Facility at the Japan Proton Accelerator Research Complex. This new diffractometer can be used for a detailed analysis of large proteins such as membrane proteins and supermolecular complex. A quantitative comparison of the intensity and pulse width of a decoupled moderator (DM) against a coupled moderator (CM) considering the pulse width time resolution indicated that the DM satisfies the criteria for our diffractometer rather than the CM. The results suggested that a characteristic feature of the DM, i.e., narrow pulse width with a short tail, is crucial for the separation of Bragg reflections from crystals with large unit cells. On the other hand, it should be noted that the weak signals from the DM are buried under the high-level background caused by the incoherent scattering of hydrogen atoms, especially, in the case of large unit cells. We propose a profile-fitting integration method combined with the energy loss functions and a background subtraction method achieved by employing the Statistics-sensitive Nonlinear Iterative Peak-clipping algorithm.
in the Materials and Life Science Experimental Facility at the Japan Proton Accelerator Research Complex. This new diffractometer can be used for a detailed analysis of large proteins such as membrane proteins and supermolecular complex. A quantitative comparison of the intensity and pulse width of a decoupled moderator (DM) against a coupled moderator (CM) considering the pulse width time resolution indicated that the DM satisfies the criteria for our diffractometer rather than the CM. The results suggested that a characteristic feature of the DM, i.e., narrow pulse width with a short tail, is crucial for the separation of Bragg reflections from crystals with large unit cells. On the other hand, it should be noted that the weak signals from the DM are buried under the high-level background caused by the incoherent scattering of hydrogen atoms, especially, in the case of large unit cells. We propose a profile-fitting integration method combined with the energy loss functions and a background subtraction method achieved by employing the Statistics-sensitive Nonlinear Iterative Peak-clipping algorithm.
Kusaka, Katsuhiro*; Hosoya, Takaaki*; Yamada, Taro*; Tomoyori, Katsuaki; Ohara, Takashi; Katagiri, Masaki*; Kurihara, Kazuo; Tanaka, Ichiro*; Niimura, Nobuo*
Journal of Synchrotron Radiation, 20(6), p.994 - 998, 2013/11
Times Cited Count:39 Percentile:85.54(Instruments & Instrumentation)The IBARAKI biological crystal diffractometer, iBIX, is a high-performance time-of-flight neutron single-crystal diffractometer for elucidating mainly the hydrogen, protonation and hydration structures of biological macromolecules in various life processes. Since the end of 2008, iBIX has been available to user's experiments supported by Ibaraki University. Since August 2012, an upgrade of the 14-existing detectors has begun and 16 new detectors have been installed for iBIX. The total measurement efficiency of the present diffractometer has been impoved by one order of magnitude from the previous one with the increasing of accelerator power. In December 2012, commissioning of the new detectors was successful, and collection of the diffraction dataset of ribonucrease A as a standard protein was attempted in order to estimate the performance of the upgraded iBIX in comparison with previous results. The resolution of diffraction data, equivalence among intensities of symmetry-related reflections and reliability of the refined structure have been improved dramatically. iBIX is expected to be one of the highest-performance neutron single-crystal diffractometers for biological macromolecules in the world.
 -thrombin-bivalirubin complex at pD 5.0; Protonation states and hydration structure of the enzyme-product complex
-thrombin-bivalirubin complex at pD 5.0; Protonation states and hydration structure of the enzyme-product complexYamada, Taro*; Kurihara, Kazuo; Onishi, Yuki*; Tamada, Taro; Tomoyori, Katsuaki; Masumi, Kenji*; Tanaka, Ichiro*; Kuroki, Ryota; Niimura, Nobuo*
Biochimica et Biophysica Acta; Proteins and Proteomics, 1834(8), p.1532 - 1538, 2013/08
Times Cited Count:20 Percentile:52.35(Biochemistry & Molecular Biology)The protonation states and hydration structures of the  -thrombin-bivalirubin complex were studied by joint XN refinement of the single crystal X-ray and neutron diffraction data at resolutions of 1.6 and 2.8
-thrombin-bivalirubin complex were studied by joint XN refinement of the single crystal X-ray and neutron diffraction data at resolutions of 1.6 and 2.8  , respectively. The atomic distances were estimated by carrying out X-ray crystallographic analysis at 1.25
, respectively. The atomic distances were estimated by carrying out X-ray crystallographic analysis at 1.25  resolution. The complex represents a model of the enzyme-product (EP) complex of
resolution. The complex represents a model of the enzyme-product (EP) complex of  -thrombin. The neutron scattering length maps around the active site suggest that the side chain of H57/H was deuterated. The joint XN refinement showed that occupancies for D
-thrombin. The neutron scattering length maps around the active site suggest that the side chain of H57/H was deuterated. The joint XN refinement showed that occupancies for D 1 and D
1 and D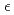 2 of H57/H were 1.0 and 0.7, respectively. However, no significant neutron scattering length density was observed around the hydroxyl oxygen O
2 of H57/H were 1.0 and 0.7, respectively. However, no significant neutron scattering length density was observed around the hydroxyl oxygen O of S195/H, which was close to the carboxylic carbon atom of dFPR-COOH. These observations suggest that the O
of S195/H, which was close to the carboxylic carbon atom of dFPR-COOH. These observations suggest that the O atom of S195/H is deprotonated and maintains its nucleophilicity in the EP complex. In addition to the active site, the hydration structures of the S1 subsite and the Exosite I, which are involved in the recognition of bivalirudin, are presented.
atom of S195/H is deprotonated and maintains its nucleophilicity in the EP complex. In addition to the active site, the hydration structures of the S1 subsite and the Exosite I, which are involved in the recognition of bivalirudin, are presented.
Yokoyama, Takeshi*; Mizuguchi, Mineyuki*; Nabeshima, Yuko*; Kusaka, Katsuhiro*; Yamada, Taro*; Hosoya, Takaaki*; Ohara, Takashi*; Kurihara, Kazuo; Tomoyori, Katsuaki*; Tanaka, Ichiro*; et al.
Journal of Structural Biology, 177(2), p.283 - 290, 2012/02
Times Cited Count:49 Percentile:82.30(Biochemistry & Molecular Biology)Tanaka, Ichiro*; Kusaka, Katsuhiro*; Hosoya, Takaaki*; Niimura, Nobuo*; Ohara, Takashi*; Kurihara, Kazuo; Yamada, Taro*; Onishi, Yuki*; Tomoyori, Katsuaki*; Yokoyama, Takeshi*
Acta Crystallographica Section D, 66(11), p.1194 - 1197, 2010/11
Times Cited Count:53 Percentile:94.69(Biochemical Research Methods)The IBARAKI Biological Crystal Diffractometer (iBIX), a new diffractometer for protein crystallography at the next-generation neutron source at J-PARC (Japan Proton Accelerator Research Complex), has been constructed and has been operational since December 2008. Preliminary structure analyses of organic crystals showed that iBIX has high performance even at 120 kW operation and the first full data set is being collected from a protein crystal.
Kurihara, Kazuo; Tomoyori, Katsuaki; Tamada, Taro; Kuroki, Ryota
no journal, ,
Molecular interaction study based on structural analysis of proteins like membrane proteins and protein complexes are an important research field in recent biological science. However, many of the target proteins have larger molecular weight and then unit cells of their crystals have larger volume, which is out of range of measurable unit cell volume for conventional diffractometers for neutron protein crystallography. Therefore, our group had designed the diffractometer which is able to cover such a crystal with large unit cell volume (target lattice length: 250 ). This proposal was accepted by Neutron Instrument Program Review Committee of J-PARC in September 2012. Larger unit cell volume causes a problem to separate spots closer to each other in spatial and time distribution in diffraction images. Therefore, our proposed diffractometer adopts longer camera distance (800 mm) and decoupled moderator as neutron source which has shorter pulse width. For covering large neutron detecting area due to long camera distance large-area detector (
). This proposal was accepted by Neutron Instrument Program Review Committee of J-PARC in September 2012. Larger unit cell volume causes a problem to separate spots closer to each other in spatial and time distribution in diffraction images. Therefore, our proposed diffractometer adopts longer camera distance (800 mm) and decoupled moderator as neutron source which has shorter pulse width. For covering large neutron detecting area due to long camera distance large-area detector ( 300 mm
300 mm  300 mm) will be developed in collaboration with Neutron Instrumentation Section in J-PARC center. The gain factor of this diffractometer is estimated to be about 20 or larger as compared with BIX-3/4 diffractometers in JRR-3 at JAEA.
300 mm) will be developed in collaboration with Neutron Instrumentation Section in J-PARC center. The gain factor of this diffractometer is estimated to be about 20 or larger as compared with BIX-3/4 diffractometers in JRR-3 at JAEA.
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
no journal, ,
Especially, large proteins such as membrane proteins or large protein complex are among the most important subjects of studies in structural biology. It will be greatly advance the fields of life science to elucidate the protein function from the structural information of hydrogen atoms and hydration waters obtained by neutron protein crystallography. Our group proposes the diffractometer which can cover such crystals with large unit cell volume (target lattice length: 250  ). We chose a decoupled liquid hydrogen moderator as an appropriate source for this high resolution diffractometer from both view points of flux and pulse width. Firstly, the problem of overlapped Bragg peaks becomes significant for high order reflections. Secondly, the large unit cell volume causes weak signals from protein crystal. The angular resolution will dominates at low angles, but time-resolution cannot be neglected with increasing the scattering angle. Consequently, the peak separation of Bragg reflection by the long flight-path will become more efficient to get over the first problem. To overcome second problem, it should be noted that the appropriate geometrical alignment of neutron supermirror guide, the selection of critical angle, and the elimination of fast neutron and
). We chose a decoupled liquid hydrogen moderator as an appropriate source for this high resolution diffractometer from both view points of flux and pulse width. Firstly, the problem of overlapped Bragg peaks becomes significant for high order reflections. Secondly, the large unit cell volume causes weak signals from protein crystal. The angular resolution will dominates at low angles, but time-resolution cannot be neglected with increasing the scattering angle. Consequently, the peak separation of Bragg reflection by the long flight-path will become more efficient to get over the first problem. To overcome second problem, it should be noted that the appropriate geometrical alignment of neutron supermirror guide, the selection of critical angle, and the elimination of fast neutron and  rays are required. We concisely estimated the instrumental resolution and intensity at sample position, and explain the result of the maximum resolvable lattice constant and measurement time.
rays are required. We concisely estimated the instrumental resolution and intensity at sample position, and explain the result of the maximum resolvable lattice constant and measurement time.
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
no journal, ,
Neutron protein crystallography is expected to contribute to the elucidation and improvement of protein function through providing structural information of hydrogen atoms and water hydration. However, recent neutron diffractometers installed at the reactor and pulsed neutron facilities does not cover proteins with a large unit cell volume, especially membrane proteins and protein complexes. From requests of structural biology community, we aim to build a high-resolution neutron time-of-flight diffractometer for biomacromolecule, which allows us to collect neutron diffraction data from crystals with unit cells even over 250  , at MLF in J-PARC. We chose a decoupled liquid hydrogen moderator as an appropriate source for this high resolution diffractometer from both view points of flux and pulse width to realize the data collection covering a full hemisphere of Bragg data to 2.0
, at MLF in J-PARC. We chose a decoupled liquid hydrogen moderator as an appropriate source for this high resolution diffractometer from both view points of flux and pulse width to realize the data collection covering a full hemisphere of Bragg data to 2.0  resolution with a lattice constant over 250
resolution with a lattice constant over 250  . The angular resolution will dominates at low angles, but becomes vanishingly small at high (back-scattering) angles. It is beneficial to take as long flight-path length L1 as possible in designing a new diffractometer for samples with large unit cells, while this comes at the expense of reduced bandwidth and at the expense of much higher cost for the instrument. Here, we introduce the general plan for the beam line components and the experimental cave, including the result of Monte Carlo simulation using the McStas software package.
. The angular resolution will dominates at low angles, but becomes vanishingly small at high (back-scattering) angles. It is beneficial to take as long flight-path length L1 as possible in designing a new diffractometer for samples with large unit cells, while this comes at the expense of reduced bandwidth and at the expense of much higher cost for the instrument. Here, we introduce the general plan for the beam line components and the experimental cave, including the result of Monte Carlo simulation using the McStas software package.
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
no journal, ,
A new diffractometer data acquisition system will be designed to adopt the distributed structure in DAQ and storage servers. The DAQ system primarily consists of a number of distributed DAQ nodes, storage servers, and of multiple clients that can retrieve and display histogram images on diffraction image viewing software. The index database that manage all distributed data location mediates between storage server (or archiving) and the multiple clients. We will introduce "DAQ-Middleware", which is the standard DAQ software in J-PARC/MLF. The dynamic device control software can concurrently control the neutron optics and sample environment devices as well as the DAQ in the instrument. The instrument control software framework called "IROHA" integrates the DAQ and device control. The compliant software regarding TOF single-crystal neutron diffraction measurements must provide a predictable user interface and functionality sufficient to review diffraction histogram data for the purpose of decision-making by ordering experimenter. Besides these functions, not only the real time (RT) display for monitoring the status of diffraction experiments but also the RT analysis including data corrections as well as peak searching, cell determination, indexing and integration functionality would be considered to be indispensable especially in order to confirm the validity of measurements.
Kurihara, Kazuo; Tomoyori, Katsuaki; Tamada, Taro; Kuroki, Ryota
no journal, ,
Many proteins, especially membrane proteins and protein complexes, have larger molecular weight and then unit cells of their crystals have larger volume. Therefore, our group had designed the diffractometer which can cover such crystals with large unit cell volume (target lattice length: 250 ) at J-PARC. In order to separate spots closer to each other in spatial as well as time dimension in diffraction images, our proposed diffractometer adopts longer camera distance (
) at J-PARC. In order to separate spots closer to each other in spatial as well as time dimension in diffraction images, our proposed diffractometer adopts longer camera distance ( 2 = 800mm) and selects decoupled hydrogen moderator as neutron source which has shorter pulse width. Under the conditions that
2 = 800mm) and selects decoupled hydrogen moderator as neutron source which has shorter pulse width. Under the conditions that  1 is 33.5m, beam divergence 0.4
1 is 33.5m, beam divergence 0.4 and crystal edge size 2mm, this diffractometer is estimated to afford the resolution (
and crystal edge size 2mm, this diffractometer is estimated to afford the resolution (
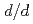 ) of 1% at the middle and high 2
) of 1% at the middle and high 2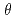
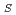 angles and be able to resolve spots diffracted from crystals with a lattice length of 220
angles and be able to resolve spots diffracted from crystals with a lattice length of 220 in each axis at
in each axis at 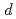 -space of 2.0
-space of 2.0 . In order to increase measurement efficiency, novel large-area detector (larger than 300mm
. In order to increase measurement efficiency, novel large-area detector (larger than 300mm  300mm) with a spatial resolution of better than 2.5mm is under development. More than 40 detectors plan to be installed, providing the total solid angle coverage of larger than 33%.
300mm) with a spatial resolution of better than 2.5mm is under development. More than 40 detectors plan to be installed, providing the total solid angle coverage of larger than 33%.
 BIX) in J-PARC
BIX) in J-PARCKurihara, Kazuo; Ohara, Takashi; Kusaka, Katsuhiro*; Niita, Koji*; Hosoya, Takaaki*; Tomoyori, Katsuaki*; Niimura, Nobuo*; Tanaka, Ichiro*
no journal, ,
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
no journal, ,
The energy struggling functions such as Vavilov and Landau distributions used to describe the energy loss of charged particles traversing a thin absorber were found to be in excellent agreement with the observed time-of-flight (TOF) profile observed by a TOF single-crystal diffractometer installed at a coupled moderator (CM) pulsed neutron source in J-PARC. It might be expected that the functions are also applicable in the case of decoupled moderator (DM) because the asymmetrical tail of the emission pulse is generated by the thermalized delayed neutron as with the CM while the pulse width of the DM is small compared with that of the CM. In the case of DM with small tail, the problem regarding the overlap of reflections might not be so serious even with large unit cell. On the other hand, it should be noted that the weak signals from biomarcromolecular crystals with the large unit cell are buried under the high level background caused by the incoherent scattering of hydrogen atoms. The profile functions especially might be useful to evaluate precisely the weak signal due to large unit cell of crystals. We will propose the profile-fitting integration method with the combined use of the energy struggling functions and background subtraction method by the Statistics-sensitive Nonlinear Iterative Peak-Clipping (SNIP) algorithm.
Tomoyori, Katsuaki
no journal, ,
We are planning to design a high-resolution biomacromolecule neutron time-of-flight (TOF) diffractometer, which allows us to collect data from crystals with the unit cells over 250 , at Materials and Life Science Experimental Facility (MLF) in Japan Proton Accelerator Research Complex (J-PARC). This new diffractometer can elucidate the detail mechanism of large proteins such as membrane proteins and supermolecular complex. The quantitative evaluation of the intensity and pulse width of a decoupled moderator (DM) against a coupled moderator (CM) considering pulse width time resolution demonstrated that a DM certainly satisfies specification of our diffractometer rather than a CM. It shows the characteristic feature of a DM, narrow pulse width with a short tail, is crucial for the separation of Bragg reflections from crystals with large unit cells. Furthermore, we proceed to design high efficient supermirror neutron guide and scintillation neutron detectors with large solid angles. We will focus on specification of new diffractometer thus obtained by our simulation and explain the performance of the new diffractometer.
, at Materials and Life Science Experimental Facility (MLF) in Japan Proton Accelerator Research Complex (J-PARC). This new diffractometer can elucidate the detail mechanism of large proteins such as membrane proteins and supermolecular complex. The quantitative evaluation of the intensity and pulse width of a decoupled moderator (DM) against a coupled moderator (CM) considering pulse width time resolution demonstrated that a DM certainly satisfies specification of our diffractometer rather than a CM. It shows the characteristic feature of a DM, narrow pulse width with a short tail, is crucial for the separation of Bragg reflections from crystals with large unit cells. Furthermore, we proceed to design high efficient supermirror neutron guide and scintillation neutron detectors with large solid angles. We will focus on specification of new diffractometer thus obtained by our simulation and explain the performance of the new diffractometer.
Kurihara, Kazuo; Tomoyori, Katsuaki; Tamada, Taro; Kuroki, Ryota
no journal, ,
Many of membrane proteins and protein complexes have larger molecular weight and then unit cells of their crystals have larger volume. Therefore, our group had designed the diffractometer which is able to cover such a crystal with large unit cell volume (target lattice length: 250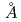 ). This proposal was accepted by Neutron Instrument Program Review Committee of J-PARC in September 2012. Larger unit cell volume causes a problem to separate spots closer to each other in spatial and time distribution in diffraction images. Therefore, our proposed diffractometer adoptted longer camera distance (800mm) and decoupled moderator as neutron source which has shorter pulse width. The neutron guide tube was designed to use limited surface of the decoupled moderator with high luminescence (40mm high
). This proposal was accepted by Neutron Instrument Program Review Committee of J-PARC in September 2012. Larger unit cell volume causes a problem to separate spots closer to each other in spatial and time distribution in diffraction images. Therefore, our proposed diffractometer adoptted longer camera distance (800mm) and decoupled moderator as neutron source which has shorter pulse width. The neutron guide tube was designed to use limited surface of the decoupled moderator with high luminescence (40mm high  60mm wide) whose luminosity is 1.24 times as high as that of average of the whole surface in the range over 2.86
60mm wide) whose luminosity is 1.24 times as high as that of average of the whole surface in the range over 2.86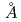 of wavelength. In addition, ellipsoidal shape in vertical design of the guide was adopted to suppress the number of neutron reflections at the guide mirror accompanying reduction of neutron intensity. In the horizontal design, curved shape was in part introduced to eliminate unnecessary
of wavelength. In addition, ellipsoidal shape in vertical design of the guide was adopted to suppress the number of neutron reflections at the guide mirror accompanying reduction of neutron intensity. In the horizontal design, curved shape was in part introduced to eliminate unnecessary  -rays and short-wavelength neutrons. According to ray-tracing simulation by McStas code, neutron flux at the sample position is estimated to be 5
-rays and short-wavelength neutrons. According to ray-tracing simulation by McStas code, neutron flux at the sample position is estimated to be 5 10
10 /cm
/cm /s in the wavelength range of 1.5
/s in the wavelength range of 1.5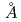 - 5.6
- 5.6 (first frame).
(first frame).
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
no journal, ,
In protein crystallography, the large biomacromolecules like the membrane proteins and protein complex are one of the most important research objects. It brings greatly contributions to the life science research field to elucidate the function by means of the structural analyses including hydrogen atom and hydration of these proteins. We propose to construct a new instrument to realize the neutron diffraction experiment using protein crystals with large unit cells. In scattering experiment with time-of-flight method, the most advantageous feature is to be able to improve the energy or time resolution by increasing flight path length. The long flight path can facilitate the spot separation from the measurement of protein crystals with large unit cells in the new instrument to be constructed in J-PARC/MLF. At present, we are predominantly planning to choose the decoupled moderator (DM) with comparatively narrow pulse width considering limited space to accommodate our new instrument inside J-PARC/MLF buildings. We evaluated the instrumental parameters to realize the measurement of protein crystal with large unit cell over 250  . The uncertainty of d-spacings is very large in low angle bank detector. It could be required to improve the angular divergence with some collimator and slit apparatus. We evaluated the capability of spot separation against scattering angles and determined the flight path length L1, L2 and the required angular divergence.
. The uncertainty of d-spacings is very large in low angle bank detector. It could be required to improve the angular divergence with some collimator and slit apparatus. We evaluated the capability of spot separation against scattering angles and determined the flight path length L1, L2 and the required angular divergence.
Tamada, Taro; Hirano, Yu; Tomoyori, Katsuaki; Kurihara, Kazuo
no journal, ,
Two facilities for neutron protein crystallography have been installed in Japan Atomic Energy Agency. One is the research reactor, JRR-3, and the other is Material and Life science experimental Facility (MLF) in J-PARC. We have performed high-resolution neutron crystal structure analyses of two electron transfer proteins. We succeeded in data collection of these proteins at higher resolution, 1.1 (high-potential iron-sulfur protein) and 1.4
(high-potential iron-sulfur protein) and 1.4 (NADH-cytochrome b5 reductase), using BL03 (iBIX) beamline in J-PARC/MLF. Joint neutron and X-ray crystallographic refinement is in progress, but we have already confirmed some characteristic hydorogens which have unideal geometries. In addition, we have a plan of installation of new diffractomer in J-PARC, which is able to cover such a crystal with large unit cell (~250
(NADH-cytochrome b5 reductase), using BL03 (iBIX) beamline in J-PARC/MLF. Joint neutron and X-ray crystallographic refinement is in progress, but we have already confirmed some characteristic hydorogens which have unideal geometries. In addition, we have a plan of installation of new diffractomer in J-PARC, which is able to cover such a crystal with large unit cell (~250 ). The operation of new diffractometer will allow neutron structure analyses of membrane proteins and protein complexes. In this presentation, we also talk about our approach for installation of new diffractomer.
). The operation of new diffractometer will allow neutron structure analyses of membrane proteins and protein complexes. In this presentation, we also talk about our approach for installation of new diffractomer.
Tanaka, Ichiro*; Ohara, Takashi; Kurihara, Kazuo; Kusaka, Katsuhiro*; Hosoya, Takaaki; Tomoyori, Katsuaki*; Niimura, Nobuo*; Ozeki, Tomoji*; Aizawa, Kazuya; Arai, Masatoshi; et al.
no journal, ,
IBARAKI Biological Crystal Diffractometer is a new single-crystal neutron diffractometer for biological and chemical crystallography, and is now being constructed at J-PARC by Ibaraki Prefectural Government in Japan. This diffractometer is designed for the protein crystals with the cell dimension up to 135  . The measurement efficiency is more than 50 times larger than the present neutron diffractometer, BIX-3/BIX-4 in JRR-3 reactor at JAEA. To achieve this performance, we have selected a coupled moderator, and worked out the optimisation of the neutron guide tube. For the detector, a new wavelength-shifting-fiber type scintillation area detector system with high spatial (0.5-1.0 mm) and time (1ms-) resolution are in development.
. The measurement efficiency is more than 50 times larger than the present neutron diffractometer, BIX-3/BIX-4 in JRR-3 reactor at JAEA. To achieve this performance, we have selected a coupled moderator, and worked out the optimisation of the neutron guide tube. For the detector, a new wavelength-shifting-fiber type scintillation area detector system with high spatial (0.5-1.0 mm) and time (1ms-) resolution are in development.