Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Auh, Y. H.*; Neal, N. N.*; Arole, K.*; Regis, N. A.*; Nguyen, T.*; Ogawa, Shuichi*; Tsuda, Yasutaka; Yoshigoe, Akitaka; Radovic, M.*; Green, M. J.*; et al.
ACS Applied Materials & Interfaces, 17(21), p.31392 - 31402, 2025/05
Times Cited Count:0 Percentile:0.00(Nanoscience & Nanotechnology)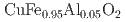
Tamatsukuri, Hiromu; Uchihara, Takeru*; Mitsuda, Setsuo*; Ishii, Yuta*; Nakao, Hironori*; Takehana, Kanji*; Imanaka, Yasutaka*
Physical Review B, 111(13), p.134403_1 - 134403_9, 2025/04
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Suzuki, Seiya; Katsube, Daiki*; Yano, Masahiro; Tsuda, Yasutaka; Terasawa, Tomoo; Ozawa, Takahiro*; Fukutani, Katsuyuki; Kim, Y.*; Asaoka, Hidehito; Yuhara, Junji*; et al.
Small Methods, 9(3), p.2400863_1 - 2400863_9, 2025/03
Times Cited Count:1 Percentile:30.18(Chemistry, Physical)Yoshigoe, Akitaka; Tsuda, Yasutaka; Kobata, Masaaki; Okane, Tetsuo; Satou, Yukihiko; Okochi, Takuo*
e-Journal of Surface Science and Nanotechnology (Internet), 23(1), p.16 - 21, 2025/02
 Cl on Cu oxide Surfaces; Cu
Cl on Cu oxide Surfaces; Cu O(111) and bulk Cu
O(111) and bulk Cu O precursor "29"; Structure on Cu(111)
O precursor "29"; Structure on Cu(111)Hayashida, Koki*; Tsuda, Yasutaka; Murase, Natsumi*; Yamada, Takashi*; Yoshigoe, Akitaka; Di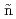 o, W. A.*; Okada, Michio*
o, W. A.*; Okada, Michio*
Applied Surface Science, 669, p.160475_1 - 160475_6, 2024/10
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)Kato, Hiroyuki S.*; Muroyama, Mizuho*; Kobayakawa, Nano*; Muneyasu, Riku*; Tsuda, Yasutaka; Murase, Natsumi*; Watanabe, Seiya*; Yamada, Takashi*; Kanematsu, Yusuke*; Tachikawa, Masanori*; et al.
Journal of Physical Chemistry Letters (Internet), 15(43), p.10769 - 10776, 2024/10
Times Cited Count:1 Percentile:37.13(Chemistry, Physical) 7
7Kakiuchi, Takuhiro*; Anai, Ryota*; Saiki, Taiju*; Tsuda, Yasutaka; Yoshigoe, Akitaka
Journal of Physical Chemistry C, 128(31), p.13052 - 13063, 2024/08
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)0xidation at the interface and the surface of Si(111) substrate with thin Hf films were studied using synchrotron radiation photoelectron spectroscopy in conjunction with supersonic oxygen molecular beams (SOMB). An Hf/Si(111) with a coverage of 0.5 monolayer (ML) included HfSi and HfSi . Following exposures to thermal oxygen molecules with a translational energy (Et) of 0.03 eV, HfSi was oxidized into Hf
. Following exposures to thermal oxygen molecules with a translational energy (Et) of 0.03 eV, HfSi was oxidized into Hf valence. Following SOMB irradiation with Et of 0.39 eV, the other HfSi
valence. Following SOMB irradiation with Et of 0.39 eV, the other HfSi could be oxidized into the Hf
could be oxidized into the Hf . Following the thermal O
. Following the thermal O exposures, the metallic Hf was nonlocally oxidized to HfO
exposures, the metallic Hf was nonlocally oxidized to HfO via trapping-mediated dissociative adsorption. Meanwhile, the segregated Si atoms were oxidized by SOMB irradiation with 2.2 eV and SiO
via trapping-mediated dissociative adsorption. Meanwhile, the segregated Si atoms were oxidized by SOMB irradiation with 2.2 eV and SiO was generated on the surface.
was generated on the surface.
Kamiya, Junichiro; Abe, Kazuhide; Fujimori, Shinichi; Fukuda, Tatsuo; Kobata, Masaaki; Morohashi, Yuko; Tsuda, Yasutaka; Yamada, Ippei; Yoshigoe, Akitaka
e-Journal of Surface Science and Nanotechnology (Internet), 22(4), p.316 - 326, 2024/08
The activation and deterioration mechanisms of the Ti-Zr-V non-evaporable getter (NEG) coating have been investigated. Operando analysis of the surface chemical composition change of the Ti-Zr-V coating was performed by the synchrotron radiation photoelectron spectroscopy (SRPES) during the process of raising the sample temperature to 250 C, corresponding to the activation process of NEG coating. The surface oxidation process was also characterized by the SRPES during the injection of O_2 gas into the chamber while keeping the sample temperature at 250
C, corresponding to the activation process of NEG coating. The surface oxidation process was also characterized by the SRPES during the injection of O_2 gas into the chamber while keeping the sample temperature at 250 C, corresponding to the deterioration process of NEG coating, i.e. surface oxidation and oxygen diffusion to the coating interior. The depth profile of the oxidized sample was measured with X-ray photoelectron spectroscopy. The results shows, in the activation process, the surface Zr gets the oxygen from the oxides of Ti and V at the first stage, resulting in the metallic Ti and V on the surface, and the oxygen of the Zr-oxide and/or Zr sub-oxides diffuse to the interior of the coating in the continuous temperature rise, resulting in the metallic Zr on the surface. It is further suggested that the deterioration of the Ti-Zr-V NEG coating means the Zr and secondary Ti are oxidized deep into the coating, resulting in the restriction of the oxygen migration from the NEG compositions on the surface and consequently the lack of surface metallization.
C, corresponding to the deterioration process of NEG coating, i.e. surface oxidation and oxygen diffusion to the coating interior. The depth profile of the oxidized sample was measured with X-ray photoelectron spectroscopy. The results shows, in the activation process, the surface Zr gets the oxygen from the oxides of Ti and V at the first stage, resulting in the metallic Ti and V on the surface, and the oxygen of the Zr-oxide and/or Zr sub-oxides diffuse to the interior of the coating in the continuous temperature rise, resulting in the metallic Zr on the surface. It is further suggested that the deterioration of the Ti-Zr-V NEG coating means the Zr and secondary Ti are oxidized deep into the coating, resulting in the restriction of the oxygen migration from the NEG compositions on the surface and consequently the lack of surface metallization.
 Cl dissociation, CH
Cl dissociation, CH abstraction, and Cl adsorption from the dissociative scattering of supersonic CH
abstraction, and Cl adsorption from the dissociative scattering of supersonic CH Cl on Cu(111) and Cu(410)
Cl on Cu(111) and Cu(410)Makino, Takamasa*; Tsuda, Yasutaka; Yoshigoe, Akitaka; Di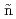 o, W. A.*; Okada, Michio*
o, W. A.*; Okada, Michio*
Applied Surface Science, 642, p.158568_1 - 158568_6, 2024/01
Times Cited Count:4 Percentile:37.63(Chemistry, Physical) by monolayer hexagonal boron nitride coating for improved photo- and thermionic-cathodes
by monolayer hexagonal boron nitride coating for improved photo- and thermionic-cathodesYamaguchi, Hisato*; Yusa, Ryunosuke*; Wang, G.*; Pettes, M. T.*; Liu, F.*; Tsuda, Yasutaka; Yoshigoe, Akitaka; Abukawa, Tadashi*; Moody, N. A.*; Ogawa, Shuichi*
Applied Physics Letters, 122(14), p.141901_1 - 141901_7, 2023/04
Times Cited Count:7 Percentile:62.75(Physics, Applied)A lowering of work function for LaB by monolayer hexagonal BN coating is reported. Photoemission electron microcopy (PEEM) and thermionic emission electron microscopy (TEEM) both revealed that the hBN coated region of a LaB
by monolayer hexagonal BN coating is reported. Photoemission electron microcopy (PEEM) and thermionic emission electron microscopy (TEEM) both revealed that the hBN coated region of a LaB (100) single crystal has lower work function compared to the bare (i.e., non-coated) and graphene coated regions. A larger decrease of work function for the hBN coated LaB
(100) single crystal has lower work function compared to the bare (i.e., non-coated) and graphene coated regions. A larger decrease of work function for the hBN coated LaB (100) compared to graphene coated LaB
(100) compared to graphene coated LaB (100) was qualitatively supported by our density functional theory (DFT) calculations. Adding an oxide layer in the calculations improved consistency between the calculation and experimental results. We followed up our calculations with synchrotron-radiation X-ray photoelectron spectroscopy (SR-XPS) and confirmed the presence of an oxide layer on our LaB
(100) was qualitatively supported by our density functional theory (DFT) calculations. Adding an oxide layer in the calculations improved consistency between the calculation and experimental results. We followed up our calculations with synchrotron-radiation X-ray photoelectron spectroscopy (SR-XPS) and confirmed the presence of an oxide layer on our LaB .
.
Tsuda, Yasutaka
Nihonkai Shimbun, P. 7, 2023/02
no abstracts in English
Ogawa, Shuichi*; Tsuda, Yasutaka; Sakamoto, Tetsuya*; Okigawa, Yuki*; Masuzawa, Tomoaki*; Yoshigoe, Akitaka; Abukawa, Tadashi*; Yamada, Takatoshi*
Applied Surface Science, 605, p.154748_1 - 154748_6, 2022/12
Times Cited Count:7 Percentile:47.60(Chemistry, Physical)Immersion of graphene in KOH solution improves its mobility on SiO /Si wafers. This is thought to be due to electron doping by modification with K atoms, but the K atom concentration C
/Si wafers. This is thought to be due to electron doping by modification with K atoms, but the K atom concentration C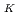 in the graphene has not been clarified yet. In this study, the C
in the graphene has not been clarified yet. In this study, the C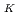 was determined by XPS analysis using high-brilliance synchrotron radiation. The time evolution of C
was determined by XPS analysis using high-brilliance synchrotron radiation. The time evolution of C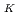 was determined by real-time observation, and the C
was determined by real-time observation, and the C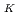 before irradiation of synchrotron radiation was estimated to be 0.94%. The C 1s spectrum shifted to the low binding energy side with the desorption of K atoms. This indicates that the electron doping concentration into graphene is decreasing, and it is experimentally confirmed that K atoms inject electrons into graphene.
before irradiation of synchrotron radiation was estimated to be 0.94%. The C 1s spectrum shifted to the low binding energy side with the desorption of K atoms. This indicates that the electron doping concentration into graphene is decreasing, and it is experimentally confirmed that K atoms inject electrons into graphene.
 species at SiO
species at SiO /Si interfaces in Si dry oxidation; Comparison between p-Si(001) and n-Si(001) surfaces
/Si interfaces in Si dry oxidation; Comparison between p-Si(001) and n-Si(001) surfacesTsuda, Yasutaka; Yoshigoe, Akitaka; Ogawa, Shuichi*; Sakamoto, Tetsuya*; Yamamoto, Yoshiki*; Yamamoto, Yukio*; Takakuwa, Yuji*
Journal of Chemical Physics, 157(23), p.234705_1 - 234705_21, 2022/12
Times Cited Count:2 Percentile:14.89(Chemistry, Physical) molecule at SiO
molecule at SiO /Si(001) interface during Si dry oxidation
/Si(001) interface during Si dry oxidationTsuda, Yasutaka; Yoshigoe, Akitaka; Ogawa, Shuichi*; Sakamoto, Tetsuya*; Takakuwa, Yuji*
e-Journal of Surface Science and Nanotechnology (Internet), 21(1), p.30 - 39, 2022/11
Tsuda, Yasutaka; Gueriba, J. S.*; Ueta, Hirokazu*; Di o, W. A.*; Kurahashi, Mitsunori*; Okada, Michio*
o, W. A.*; Kurahashi, Mitsunori*; Okada, Michio*
JACS Au (Internet), 2(8), p.1839 - 1847, 2022/08
 O vapor on GaN surfaces
O vapor on GaN surfacesSumiya, Masatomo*; Sumita, Masato*; Tsuda, Yasutaka; Sakamoto, Tetsuya; Sang, L.*; Harada, Yoshitomo*; Yoshigoe, Akitaka
Science and Technology of Advanced Materials, 23(1), p.189 - 198, 2022/00
Times Cited Count:6 Percentile:39.26(Materials Science, Multidisciplinary)GaN is an attracting material for power-electronic devices. Understanding the oxidation at GaN surface is important for improving metal-oxide-semiconductor (MOS) devices. In this study, the oxidation at GaN surfaces depending on the GaN crystal planes (+c, -c, and m-plane) was investigated by real time XPS and DFT-MD simulation. We found that H O vapor has the highest reactivity due to the spin interaction between H
O vapor has the highest reactivity due to the spin interaction between H O and GaN surfaces. The bond length between the Ga and N on the -c GaN surface was increased by OH attacking the back side of three-fold Ga atom. The chemisorption on the m-plane was dominant. The intense reactions of oxidation and Al
O and GaN surfaces. The bond length between the Ga and N on the -c GaN surface was increased by OH attacking the back side of three-fold Ga atom. The chemisorption on the m-plane was dominant. The intense reactions of oxidation and Al Ga
Ga N formation for p-GaN were observed at the interface of the Al
N formation for p-GaN were observed at the interface of the Al O
O layer deposited by ALD using H
layer deposited by ALD using H O vapor. This study suggests that an oxidant gas other than H
O vapor. This study suggests that an oxidant gas other than H O and O
O and O should be used to avoid unintentional oxidation during Al
should be used to avoid unintentional oxidation during Al Ga
Ga N atomi layer deposition.
N atomi layer deposition.
 molecular adsorption on Cu(111); Copper oxides
molecular adsorption on Cu(111); Copper oxidesHayashida, Koki*; Tsuda, Yasutaka; Yamada, Takashi*; Yoshigoe, Akitaka; Okada, Michio*
ACS Omega (Internet), 6(40), p.26814 - 26820, 2021/10
Times Cited Count:13 Percentile:54.62(Chemistry, Multidisciplinary)We report the X-ray photoemission spectroscopy (XPS) characterization of the bulk Cu O(111) surface and 8-type and 29-type oxide structures on Cu(111) prepared by using 0.5 eV O
O(111) surface and 8-type and 29-type oxide structures on Cu(111) prepared by using 0.5 eV O supersonic molecular beam (SSMB) source. We propose a new structural model for the 8-type oxide structure and also confirmed the previously proposed model for the [29] oxide structure on Cu(111), based on the O1s XPS spectra. The detection-angle dependence of the O 1s spectra supports that the nanopyramidal model is more preferable for the (
supersonic molecular beam (SSMB) source. We propose a new structural model for the 8-type oxide structure and also confirmed the previously proposed model for the [29] oxide structure on Cu(111), based on the O1s XPS spectra. The detection-angle dependence of the O 1s spectra supports that the nanopyramidal model is more preferable for the (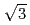 X
X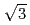 )R30
)R30 Cu
Cu O(111). We also report the electronic excitations which O1s electrons suffer.
O(111). We also report the electronic excitations which O1s electrons suffer.
 (001) surface using supersonic seeded oxygen molecular beam
(001) surface using supersonic seeded oxygen molecular beamKatsube, Daiki*; Ono, Shinya*; Takayanagi, Shuhei*; Ojima, Shoki*; Maeda, Motoyasu*; Origuchi, Naoki*; Ogawa, Arata*; Ikeda, Natsuki*; Aoyagi, Yoshihide*; Kabutoya, Yuito*; et al.
Langmuir, 37(42), p.12313 - 12317, 2021/10
Times Cited Count:4 Percentile:19.51(Chemistry, Multidisciplinary)We investigated the oxidation of oxygen vacancies at the surface of anatase TiO (001) using supersonic seeded molecular beam (SSMB) of oxygen. The oxygen vacancies at the top-surface and sub-surface could be eliminated by the supply of oxygen using an SSMB. These results indicate that the interstitial vacancies can be mostly assigned to oxygen vacancies, which can be effectively eliminated by using an oxygen SSMB. Oxygen vacancies are present on the surface of anatase TiO
(001) using supersonic seeded molecular beam (SSMB) of oxygen. The oxygen vacancies at the top-surface and sub-surface could be eliminated by the supply of oxygen using an SSMB. These results indicate that the interstitial vacancies can be mostly assigned to oxygen vacancies, which can be effectively eliminated by using an oxygen SSMB. Oxygen vacancies are present on the surface of anatase TiO (001) when it is untreated before transfer to a vacuum chamber. These vacancies, which are stable in the as-grown condition, could also be effectively eliminated using the oxygen SSMB.
(001) when it is untreated before transfer to a vacuum chamber. These vacancies, which are stable in the as-grown condition, could also be effectively eliminated using the oxygen SSMB.
 O formation on Cu
O formation on Cu Pd(111) and Cu
Pd(111) and Cu Pt(111)
Pt(111)Tsuda, Yasutaka; Gueriba, J. S.*; Makino, Takamasa*; Di o, W. A.*; Yoshigoe, Akitaka; Okada, Michio*
o, W. A.*; Yoshigoe, Akitaka; Okada, Michio*
Scientific Reports (Internet), 11, p.3906_1 - 3906_8, 2021/02
Times Cited Count:7 Percentile:26.10(Multidisciplinary Sciences)Okada, Michio*; Tsuda, Yasutaka*; Yoshigoe, Akitaka; Di o, W. A.*
o, W. A.*
Do To Dogokin, 56(1), p.232 - 236, 2017/00
We reported the our studies on the surface temperature (Ts) dependence of oxidation on the Cu Au(111) surface by a supersonic O
Au(111) surface by a supersonic O molecular beam, using synchrotron radiation X-ray photoemission spectroscopy. Clean surface shows strong Au segregation to the top layer, i.e., Au surface enrichment of the clean surface. Complete Cu segregation to the surface occurs at 0.5 ML O surface coverage. The Au-rich second and third layers of the oxidized surface demonstrate the protective layer formation against oxidation deeper into the bulk. We found that Cu
molecular beam, using synchrotron radiation X-ray photoemission spectroscopy. Clean surface shows strong Au segregation to the top layer, i.e., Au surface enrichment of the clean surface. Complete Cu segregation to the surface occurs at 0.5 ML O surface coverage. The Au-rich second and third layers of the oxidized surface demonstrate the protective layer formation against oxidation deeper into the bulk. We found that Cu O formation occurs. At Ts = 300K, the Cu
O formation occurs. At Ts = 300K, the Cu O growth is not so effective. The surface oxidation is less effective on Cu
O growth is not so effective. The surface oxidation is less effective on Cu Au(111) than on Cu(111). At Ts = 400K, the protection by the Au-rich layer against oxidation into bulk is effective. At Ts = 500K, the Au protective layer is broken due to effective Au diffusion and thus Cu
Au(111) than on Cu(111). At Ts = 400K, the protection by the Au-rich layer against oxidation into bulk is effective. At Ts = 500K, the Au protective layer is broken due to effective Au diffusion and thus Cu O grows deeper into bulk.
O grows deeper into bulk.