Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
Chemistry Letters, 50(6), p.1169 - 1172, 2021/06
Times Cited Count:2 Percentile:14.19(Chemistry, Multidisciplinary)The electrochemical behavior of uranium (IV) tetrachloride in ionic liquid-DMF mixture was studied for first time in order to build a redox flow battery (RFB) using U as an electrode active material. We found a quasi-reversible U /U
/U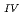 couple that could be applied to the anode reaction of the RFB.
couple that could be applied to the anode reaction of the RFB.
Tsuji, Tomoyuki; Sugitsue, Noritake; Sato, Fuminori; Matsushima, Ryotatsu; Kataoka, Shoji; Okada, Shota; Sasaki, Toshiki; Inoue, Junya
Nihon Genshiryoku Gakkai-Shi ATOMO , 62(11), p.658 - 663, 2020/11
, 62(11), p.658 - 663, 2020/11
no abstracts in English
 He
He
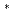 excimer cluster tracers in superfluid helium-4 via neutron-
excimer cluster tracers in superfluid helium-4 via neutron- He absorption reaction
He absorption reactionSonnenschein, V.*; Tsuji, Yoshiyuki*; Kokuryu, Shoma*; Kubo, Wataru*; Suzuki, So*; Tomita, Hideki*; Kiyanagi, Yoshiaki*; Iguchi, Tetsuo*; Matsushita, Taku*; Wada, Nobuo*; et al.
Review of Scientific Instruments, 91(3), p.033318_1 - 033318_12, 2020/03
Times Cited Count:0 Percentile:0(Instruments & Instrumentation) He
He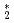 clusters via neutron-
clusters via neutron- He absorption reaction toward visualization of full velocity field in quantum turbulence
He absorption reaction toward visualization of full velocity field in quantum turbulenceMatsushita, Taku*; Sonnenschein, V.*; Guo, W.*; Hayashida, Hirotoshi*; Hiroi, Kosuke; Hirota, Katsuya*; Iguchi, Tetsuo*; Ito, Daisuke*; Kitaguchi, Masaaki*; Kiyanagi, Yoshiaki*; et al.
Journal of Low Temperature Physics, 196(1-2), p.275 - 282, 2019/07
Times Cited Count:1 Percentile:4.65(Physics, Applied)Nakamura, Yoshihiko*; Shibata, Akinobu*; Gong, W.*; Harjo, S.; Kawasaki, Takuro; Ito, Atsushi*; Tsuji, Nobuhiro*
Proceedings of International Conference on Martensitic Transformations: Chicago, p.155 - 158, 2018/04
Oe, Kazuhiro*; Attallah, M. F.*; Asai, Masato; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke*; Kasamatsu, Yoshitaka*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1317 - 1320, 2015/02
Times Cited Count:10 Percentile:64.26(Chemistry, Analytical)A new technique for continuous dissolution of nuclear reaction products transported by a gas-jet system was developed for superheavy element (SHE) chemistry. In this technique, a hydrophobic membrane is utilized to separate an aqueous phase from the gas phase. With this technique, the dissolution efficiencies of short-lived radionuclides of 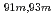 Mo and
Mo and 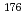 W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
 /Md
/Md reduction potential studied with flow electrolytic chromatography
reduction potential studied with flow electrolytic chromatographyToyoshima, Atsushi; Li, Z.*; Asai, Masato; Sato, Nozomi; Sato, Tetsuya; Kikuchi, Takahiro; Kaneya, Yusuke; Kitatsuji, Yoshihiro; Tsukada, Kazuaki; Nagame, Yuichiro; et al.
Inorganic Chemistry, 52(21), p.12311 - 12313, 2013/11
Times Cited Count:6 Percentile:23.49(Chemistry, Inorganic & Nuclear)The reduction behavior of mendelevium (Md) was studied using a flow electrolytic chromatography apparatus. By applying appropriate potentials on the chromatography column, the more stable Md is reduced to Md
is reduced to Md . The reduction potential of the Md
. The reduction potential of the Md + e
+ e
 Md
Md couple was determined to be -0.16
couple was determined to be -0.16 0.05 V vs. a normal hydrogen electrode.
0.05 V vs. a normal hydrogen electrode.
Hoshi, Akiko; Kameo, Yutaka; Katayama, Atsushi; Sakai, Akihiro; Tsuji, Tomoyuki; Nakashima, Mikio; Kihara, Shinji; Takahashi, Kuniaki
JAEA-Data/Code 2009-023, 84 Pages, 2010/03
In order to establish the practical evaluation methods such as scaling factor method to determine the radioactivity concentrations of the important nuclides for safety assessment of disposal of radioactive wastes, we analyzed low-level radioactive liquid waste (56 samples), which is generated from various research facilities at Nuclear Science Research Institute from FY1998 to FY2007 and accumulated the radioactivity concentrations data (563 data) of the 17 important nuclides. We investigated the correlation of the radioactivity concentrations of the important nuclides with the "Key nuclides ( Co or
Co or  Cs)". In present paper, the radioactivity concentrations data of the 17 important nuclides and the results of the correlation of the radioactivity concentrations are summarized for the data to establish the practical evaluation methods to determine the radioactivity concentrations in asphalt-solidified or cement-solidified products.
Cs)". In present paper, the radioactivity concentrations data of the 17 important nuclides and the results of the correlation of the radioactivity concentrations are summarized for the data to establish the practical evaluation methods to determine the radioactivity concentrations in asphalt-solidified or cement-solidified products.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Haba, Hiromitsu*; Oe, Kazuhiro*; et al.
Journal of the American Chemical Society, 131(26), p.9180 - 9181, 2009/07
Times Cited Count:15 Percentile:45.51(Chemistry, Multidisciplinary)We report here on the successful oxidation of element 102, nobelium (No), on an atom-at-a-time scale in 0.1 M  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No
-HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No is oxidized to No
is oxidized to No within 3 min and that the oxidized No complex with
within 3 min and that the oxidized No complex with  -HIB holds the trivalent state in the column above the applied potential of 1.0 V.
-HIB holds the trivalent state in the column above the applied potential of 1.0 V.
Suzuki, Michiyo; Sakashita, Tetsuya; Yanase, Sumino*; Kikuchi, Masahiro; Oba, Hirofumi; Higashitani, Atsushi*; Hamada, Nobuyuki*; Funayama, Tomoo; Fukamoto, Kana; Tsuji, Toshio*; et al.
Journal of Radiation Research, 50(2), p.119 - 125, 2009/04
Times Cited Count:8 Percentile:29.48(Biology)Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Kitatsuji, Yoshihiro; Tsukada, Kazuaki; Haba, Hiromitsu*; Shinohara, Atsushi*; Nagame, Yuichiro
Radiochimica Acta, 96(6), p.323 - 326, 2008/06
Times Cited Count:12 Percentile:59.06(Chemistry, Inorganic & Nuclear)We developed a new apparatus for the study of electrochemical properties of the heaviest elements. The apparatus is based on a flow electrolytic cell combined with column chromatography. Glassy-carbon fibers modified with Nafion perfluorinated cation-exchange resin are used as a working electrode as well as a cation-exchanger. The elution behavior of 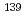 Ce with the number of 10
Ce with the number of 10 atoms in 0.1 M ammonium
atoms in 0.1 M ammonium  -hydroxyisobutyric acid solution from the column electrode was investigated at the applied potentials of 0.2 - 1.0 V versus the Ag/Agcl reference electrode in 1.0 M LiCl. It was found that
-hydroxyisobutyric acid solution from the column electrode was investigated at the applied potentials of 0.2 - 1.0 V versus the Ag/Agcl reference electrode in 1.0 M LiCl. It was found that 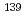 Ce
Ce is successfully oxidized to
is successfully oxidized to 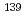 Ce
Ce even with tracer concentration at around the redox potential determined by cyclic voltammetry for the macro amounts of Ce with 10
even with tracer concentration at around the redox potential determined by cyclic voltammetry for the macro amounts of Ce with 10 atoms (10
atoms (10 M). The present oxidation reaction and separation of Ce
M). The present oxidation reaction and separation of Ce was accomplished within a few minutes.
was accomplished within a few minutes.
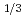 NbS
NbS
Guo, F. Z.*; Matsushita, Tomohiro*; Kobayashi, Keisuke*; Matsui, Fumihiko*; Kato, Yukako*; Daimon, Hiroshi*; Koyano, Mikio*; Yamamura, Yasuhisa*; Tsuji, Toshihide*; Saito, Yuji
Journal of Applied Physics, 99(2), p.024907_1 - 024907_3, 2006/01
Times Cited Count:8 Percentile:31.23(Physics, Applied)Stereoatomscope was used to study the atomic arrangements of intercalation compound Fe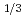 NbS
NbS . The three-dimensional atomic arrangements around different kinds of atoms (Nb and Fe) are visualized by taking the photoelectron angular distribution (PEAD) patterns at clockwise and counterclockwise circularly polarized lights. Atomic distances between the emitters and the scatterers are obtained from the PEAD patterns by measuring the rotation angles of the forward focusing peaks. The applications of stereoatomscope to intercalation compound show the possibility to build an ultimate microscope for scientist.
. The three-dimensional atomic arrangements around different kinds of atoms (Nb and Fe) are visualized by taking the photoelectron angular distribution (PEAD) patterns at clockwise and counterclockwise circularly polarized lights. Atomic distances between the emitters and the scatterers are obtained from the PEAD patterns by measuring the rotation angles of the forward focusing peaks. The applications of stereoatomscope to intercalation compound show the possibility to build an ultimate microscope for scientist.
 NbS
NbS
Saito, Yuji; Kobayashi, Keisuke*; Fujimori, Atsushi; Yamamura, Yasuhisa*; Koyano, Mikio*; Tsuji, Toshihide*; Katayama, Shinichi*
Journal of Electron Spectroscopy and Related Phenomena, 144-147, p.829 - 832, 2005/06
Times Cited Count:5 Percentile:27.33(Spectroscopy)no abstracts in English
Kando, Masaki; Masuda, Shinichi; Zhidkov, A.*; Yamazaki, Atsushi; Kotaki, Hideyuki; Kondo, Shuji; Homma, Takayuki*; Kanazawa, Shuhei; Nakajima, Kazuhisa; Hayashi, Yukio; et al.
Physical Review E, 71(1), p.015403_1 - 015403_4, 2005/01
Times Cited Count:33 Percentile:77.25(Physics, Fluids & Plasmas)no abstracts in English
*; Ando, Toshiro; ; Arai, Takashi; Neyatani, Yuzuru; Yoshino, Ryuji; Tsuji, Shunji; Yagyu, Junichi; Kaminaga, Atsushi; ; et al.
Journal of Nuclear Materials, 220-222, p.390 - 394, 1995/00
Times Cited Count:16 Percentile:80.76(Materials Science, Multidisciplinary)no abstracts in English
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Kitatsuji, Yoshihiro; Tsukada, Kazuaki; Ishii, Yasuo; Tome, Hayato; Asai, Masato; Haba, Hiromitsu*; Akiyama, Kazuhiko*; Oe, Kazuhiro*; et al.
no journal, ,
no abstracts in English
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Haba, Hiromitsu*; Ishii, Yasuo; Tome, Hayato; Asai, Masato; Akiyama, Kazuhiko*; Oe, Kazuhiro*; et al.
no journal, ,
Electrochemical oxidation of nobelium (No) produced in the  Cm(
Cm( C, 5n)
C, 5n) No reaction was studied using a new electrochemistry apparatus combined with chromatographic separation technique. Chromatographic behaviour of
No reaction was studied using a new electrochemistry apparatus combined with chromatographic separation technique. Chromatographic behaviour of  No in 0.1 M
No in 0.1 M  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) on the electrode surface was measured with verifying the difference in behaviour between divalent
-HIB) on the electrode surface was measured with verifying the difference in behaviour between divalent 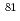 Sr
Sr and trivalent
and trivalent 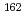 Yb
Yb ions. Independently of the applied potentials, Yb
ions. Independently of the applied potentials, Yb was eluted in the 0.1 M
was eluted in the 0.1 M  -HIB while Sr
-HIB while Sr was adsorbed on the electrode. At the low potential of 0.2 V,
was adsorbed on the electrode. At the low potential of 0.2 V,  No was adsorbed on the electrode, indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V,
No was adsorbed on the electrode, indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V,  No was unambiguously detected in the 0.1 M
No was unambiguously detected in the 0.1 M  -HIB, showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No
-HIB, showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No to No
to No is successfully performed.
is successfully performed.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Haba, Hiromitsu*; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Oe, Kazuhiro*; et al.
no journal, ,
We present a characterization technique for heavy actinides with electrochemistry. An electrochemistry apparatus combined with a chromatographic separation technique was developed to identify oxidation states of the heavy actinides on an atom-at-a-time scale. Oxidation states of nobelium (No) in aqueous solution were studied using the apparatus. Nobelium-255 with a half-life of 3.1 min was produced in the  Cm(
Cm( C, 5n) reaction at the JAEA tandem accelerator. Chromatographic behavior of No on a chemically modified electrode with Nafion perfluoronated ion-exchange resin was investigated in ammonium
C, 5n) reaction at the JAEA tandem accelerator. Chromatographic behavior of No on a chemically modified electrode with Nafion perfluoronated ion-exchange resin was investigated in ammonium  -hydroxyisobutyric acid solution. It has been found that No is bound in the most stable divalent state at low applied potentials while it exists as the trivalent ion at higher potentials, showing the electrochemical oxidation of No.
-hydroxyisobutyric acid solution. It has been found that No is bound in the most stable divalent state at low applied potentials while it exists as the trivalent ion at higher potentials, showing the electrochemical oxidation of No.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Haba, Hiromitsu*; Asai, Masato; Ishii, Yasuo; Tome, Hayato; Akiyama, Kazuhiko*; Oe, Kazuhiro*; et al.
no journal, ,
Electrochemical oxidation of element 102, nobelium (No), on an atom-at-a-time scale is presented. We developed a chemically modified electrode with perfluorinated ion-exchange resin to identify oxidation states of No based on chromatographic behavior. Nobelium-255 produced in the  Cm(
Cm( C,5n) reaction at the JAEA tandem accelerator was transported by a He/KCl gas-jet method to a chemical device. Elution behavior of
C,5n) reaction at the JAEA tandem accelerator was transported by a He/KCl gas-jet method to a chemical device. Elution behavior of  No on the electrode in
No on the electrode in  -hydroxyisobutyric acid was studied as a function of an applied potential. At the low potential of 0.2 V, chemical behavior of
-hydroxyisobutyric acid was studied as a function of an applied potential. At the low potential of 0.2 V, chemical behavior of  No was the same as that of Sr
No was the same as that of Sr , indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V, elution behavior of
, indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V, elution behavior of  No was similar to that of Yb
No was similar to that of Yb , showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No
, showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No to No
to No is successfully performed.
is successfully performed.
Satoh, Daiki; Sato, Kaoru; Takahashi, Fumiaki; Endo, Akira; Miyahara, Nobuyuki*; Tsuji, Atsushi*; Omachi, Yasushi*
no journal, ,
The present study intends to calculate the organ doses and analyze the characteristics of the radiation field inside the bodies of a mouse and human. The voxel phantom of mouse had already been developed for an 8-week-old C H/HeNs mouse in the previous work. We upgraded this phantom to improve the resolution (voxel size: 0.1
H/HeNs mouse in the previous work. We upgraded this phantom to improve the resolution (voxel size: 0.1 0.1
0.1 0.1 mm
0.1 mm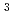 ), and add the nine solid organs. The JM phantom is the voxel phantom of a Japanese adult male developed for the analysis of internal exposure from photons and electrons. We have converted the JM phantom to use in the dose calculation on external neutron exposure, and verified it through the calculation of the absorbed dose per unit neutron fluence at each organ for monoenergetic neutrons. The calculation results showed a good agreement with the reference values reported by ICRP. From the comparison between the organ doses of the mouse and human, it was found that the relativistic dose contribution of electron in the human body is greater than that in the mouse body. This is because the neutrons are moderated inside a large receptor such as the human body, and causes the thermal neutron capture reaction that generates
), and add the nine solid organs. The JM phantom is the voxel phantom of a Japanese adult male developed for the analysis of internal exposure from photons and electrons. We have converted the JM phantom to use in the dose calculation on external neutron exposure, and verified it through the calculation of the absorbed dose per unit neutron fluence at each organ for monoenergetic neutrons. The calculation results showed a good agreement with the reference values reported by ICRP. From the comparison between the organ doses of the mouse and human, it was found that the relativistic dose contribution of electron in the human body is greater than that in the mouse body. This is because the neutrons are moderated inside a large receptor such as the human body, and causes the thermal neutron capture reaction that generates  ray and consequently electron.
ray and consequently electron.