Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tsuji, Hayato*; Nakahata, Masaki*; Hishida, Mafumi*; Seto, Hideki*; Motokawa, Ryuhei; Inoue, Takeru*; Egawa, Yasunobu*
Journal of Physical Chemistry Letters (Internet), 14(49), p.11235 - 11241, 2023/12
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Goto, Minoru; Demachi, Kazuyuki*; Ueta, Shohei; Nakano, Masaaki*; Honda, Masaki*; Tachibana, Yukio; Inaba, Yoshitomo; Aihara, Jun; Fukaya, Yuji; Tsuji, Nobumasa*; et al.
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.507 - 513, 2015/09
A concept of a plutonium burner HTGR named as Clean Burn, which has a high nuclear proliferation resistance, had been proposed by Japan Atomic Energy Agency. In addition to the high nuclear proliferation resistance, in order to enhance the safety, we propose to introduce PuO -YSZ TRISO fuel with ZrC coating to the Clean Burn. In this study, we conduct fabrication tests aiming to establish the basic technologies for fabrication of PuO
-YSZ TRISO fuel with ZrC coating to the Clean Burn. In this study, we conduct fabrication tests aiming to establish the basic technologies for fabrication of PuO -YSZ TRISO fuel with ZrC coating. Additionally, we conduct a quantitative evaluation of the security for the safety, a design of the fuel and the reactor core, and a safety evaluation for the Clean Burn to confirm the feasibility. This study is conducted by The University of Tokyo, Japan Atomic Energy Agency, Fuji Electric Co., Ltd., and Nuclear Fuel Industries, Ltd. It was started in FY2014 and will be completed in FY2017, and the first year of the implementation was on schedule.
-YSZ TRISO fuel with ZrC coating. Additionally, we conduct a quantitative evaluation of the security for the safety, a design of the fuel and the reactor core, and a safety evaluation for the Clean Burn to confirm the feasibility. This study is conducted by The University of Tokyo, Japan Atomic Energy Agency, Fuji Electric Co., Ltd., and Nuclear Fuel Industries, Ltd. It was started in FY2014 and will be completed in FY2017, and the first year of the implementation was on schedule.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Haba, Hiromitsu*; Oe, Kazuhiro*; et al.
Journal of the American Chemical Society, 131(26), p.9180 - 9181, 2009/07
Times Cited Count:15 Percentile:45.51(Chemistry, Multidisciplinary)We report here on the successful oxidation of element 102, nobelium (No), on an atom-at-a-time scale in 0.1 M  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No
-HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No is oxidized to No
is oxidized to No within 3 min and that the oxidized No complex with
within 3 min and that the oxidized No complex with  -HIB holds the trivalent state in the column above the applied potential of 1.0 V.
-HIB holds the trivalent state in the column above the applied potential of 1.0 V.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Kitatsuji, Yoshihiro; Tsukada, Kazuaki; Ishii, Yasuo; Tome, Hayato; Asai, Masato; Haba, Hiromitsu*; Akiyama, Kazuhiko*; Oe, Kazuhiro*; et al.
no journal, ,
no abstracts in English
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Haba, Hiromitsu*; Ishii, Yasuo; Tome, Hayato; Asai, Masato; Akiyama, Kazuhiko*; Oe, Kazuhiro*; et al.
no journal, ,
Electrochemical oxidation of nobelium (No) produced in the  Cm(
Cm( C, 5n)
C, 5n) No reaction was studied using a new electrochemistry apparatus combined with chromatographic separation technique. Chromatographic behaviour of
No reaction was studied using a new electrochemistry apparatus combined with chromatographic separation technique. Chromatographic behaviour of  No in 0.1 M
No in 0.1 M  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) on the electrode surface was measured with verifying the difference in behaviour between divalent
-HIB) on the electrode surface was measured with verifying the difference in behaviour between divalent 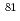 Sr
Sr and trivalent
and trivalent 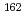 Yb
Yb ions. Independently of the applied potentials, Yb
ions. Independently of the applied potentials, Yb was eluted in the 0.1 M
was eluted in the 0.1 M  -HIB while Sr
-HIB while Sr was adsorbed on the electrode. At the low potential of 0.2 V,
was adsorbed on the electrode. At the low potential of 0.2 V,  No was adsorbed on the electrode, indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V,
No was adsorbed on the electrode, indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V,  No was unambiguously detected in the 0.1 M
No was unambiguously detected in the 0.1 M  -HIB, showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No
-HIB, showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No to No
to No is successfully performed.
is successfully performed.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Haba, Hiromitsu*; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Oe, Kazuhiro*; et al.
no journal, ,
We present a characterization technique for heavy actinides with electrochemistry. An electrochemistry apparatus combined with a chromatographic separation technique was developed to identify oxidation states of the heavy actinides on an atom-at-a-time scale. Oxidation states of nobelium (No) in aqueous solution were studied using the apparatus. Nobelium-255 with a half-life of 3.1 min was produced in the  Cm(
Cm( C, 5n) reaction at the JAEA tandem accelerator. Chromatographic behavior of No on a chemically modified electrode with Nafion perfluoronated ion-exchange resin was investigated in ammonium
C, 5n) reaction at the JAEA tandem accelerator. Chromatographic behavior of No on a chemically modified electrode with Nafion perfluoronated ion-exchange resin was investigated in ammonium  -hydroxyisobutyric acid solution. It has been found that No is bound in the most stable divalent state at low applied potentials while it exists as the trivalent ion at higher potentials, showing the electrochemical oxidation of No.
-hydroxyisobutyric acid solution. It has been found that No is bound in the most stable divalent state at low applied potentials while it exists as the trivalent ion at higher potentials, showing the electrochemical oxidation of No.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Haba, Hiromitsu*; Asai, Masato; Ishii, Yasuo; Tome, Hayato; Akiyama, Kazuhiko*; Oe, Kazuhiro*; et al.
no journal, ,
Electrochemical oxidation of element 102, nobelium (No), on an atom-at-a-time scale is presented. We developed a chemically modified electrode with perfluorinated ion-exchange resin to identify oxidation states of No based on chromatographic behavior. Nobelium-255 produced in the  Cm(
Cm( C,5n) reaction at the JAEA tandem accelerator was transported by a He/KCl gas-jet method to a chemical device. Elution behavior of
C,5n) reaction at the JAEA tandem accelerator was transported by a He/KCl gas-jet method to a chemical device. Elution behavior of  No on the electrode in
No on the electrode in  -hydroxyisobutyric acid was studied as a function of an applied potential. At the low potential of 0.2 V, chemical behavior of
-hydroxyisobutyric acid was studied as a function of an applied potential. At the low potential of 0.2 V, chemical behavior of  No was the same as that of Sr
No was the same as that of Sr , indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V, elution behavior of
, indicating that No is bound in the most stable divalent state. On the other hand, at the higher potential of 1.2 V, elution behavior of  No was similar to that of Yb
No was similar to that of Yb , showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No
, showing that No exists as a trivalent ion. These results demonstrate that the electrochemical oxidation of No to No
to No is successfully performed.
is successfully performed.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Ishii, Yasuo; Tome, Hayato*; Asai, Masato; Nagame, Yuichiro; Haba, Hiromitsu*; Akiyama, Kazuhiko*; et al.
no journal, ,
no abstracts in English