Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
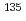 Cs/
Cs/ Cs isotope ratio
Cs isotope ratioShimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Takeda, Seiji
Journal of Radioanalytical and Nuclear Chemistry, 333(12), p.6297 - 6310, 2024/12
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Iwamoto, Toshihiro; Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
Mechanical Engineering Journal (Internet), 11(2), p.23-00444_1 - 23-00444_7, 2024/04
Arai, Yoichi; Watanabe, So; Hasegawa, Kenta; Okamura, Nobuo; Watanabe, Masayuki; Takeda, Keisuke*; Fukumoto, Hiroki*; Ago, Tomohiro*; Hagura, Naoto*; Tsukahara, Takehiko*
Nuclear Instruments and Methods in Physics Research B, 542, p.206 - 213, 2023/09
Times Cited Count:1 Percentile:25.07(Instruments & Instrumentation)Iwamoto, Toshihiro; Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
Proceedings of 30th International Conference on Nuclear Engineering (ICONE30) (Internet), 4 Pages, 2023/05
Applicability of temperature swing extraction technology employing monoamides was examined for uranium contaminated waste treatment procedure. Separation experiments on simulated target solution with three kinds of monoamides with different structure showed that Ce(IV) in the solution was selectively recovered by the temperature swing extraction operation. Based on the experiments, an appropriate monoamide for the procedure was selected.
Ouchi, Kazuki; Tsukahara, Takehiko*; Brandt, A.*; Muto, Yuki*; Nabatame, Nozomi*; Kitatsuji, Yoshihiro
Analytical Sciences, 37(12), p.1789 - 1794, 2021/12
Times Cited Count:1 Percentile:3.74(Chemistry, Analytical)We attempted to scale down a separation process of uranium (U) using the microchip column loaded with anion exchange resin to develop safety and waste-reducing separation technique. The ideal separation performance of U was obtained by the properly design of a microchannel. The concentration of U in seawater as a real-world sample could be quantified with the prepared microchip column. It indicates that the microchip column is sufficiently practical. Compared to separation of U with a general column, the column size was successfully scaled down to  1/5000.
1/5000.
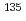 Cs/
Cs/ Cs isotopic ratio in soil collected near Fukushima Daiichi Nuclear Power Station through mass spectrometry
Cs isotopic ratio in soil collected near Fukushima Daiichi Nuclear Power Station through mass spectrometryShimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Kim, M. S.*; Shimada, Taro; Takeda, Seiji; Yamaguchi, Tetsuji
Journal of Nuclear Science and Technology, 58(11), p.1184 - 1194, 2021/11
Times Cited Count:7 Percentile:61.36(Nuclear Science & Technology)Determining the completeness of nuclear reactor decommissioning is an important step in safely utilizing nuclear power. For example,  Cs from the Fukushima Daiichi Nuclear Power Station (FDNPS) accident can be treated as background radioactivity, so determining the origin of
Cs from the Fukushima Daiichi Nuclear Power Station (FDNPS) accident can be treated as background radioactivity, so determining the origin of  Cs is essential. To accomplish this, measuring the
Cs is essential. To accomplish this, measuring the 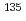 Cs/
Cs/ Cs isotope ratio can be useful, so this study optimized a solvent extraction method, with calix[4]arene-bis(t-octylbenzo-crown-6) [BOBCalixC6] in 1-octanol, to purify radioactive Cs, radiocesium, from a solution of major environmental soil elements and mass spectrometry interference elements. This optimized method was applied to Cs purification in soil samples (40 g), and the final solutions contained a total of 10
Cs isotope ratio can be useful, so this study optimized a solvent extraction method, with calix[4]arene-bis(t-octylbenzo-crown-6) [BOBCalixC6] in 1-octanol, to purify radioactive Cs, radiocesium, from a solution of major environmental soil elements and mass spectrometry interference elements. This optimized method was applied to Cs purification in soil samples (40 g), and the final solutions contained a total of 10 g/ml of the major soil elements and ng/ml concentrations at most of interfering elements. Soil samples collected near the FDNPS were then purified, and the
g/ml of the major soil elements and ng/ml concentrations at most of interfering elements. Soil samples collected near the FDNPS were then purified, and the 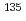 Cs/
Cs/ Cs isotope ratios were measured, using both thermal ionization mass spectrometry (TIMS) and triple quadrupole induced coupled plasma mass spectrometry (ICP-QQQ). The results of each of these measurements were compared, and we found that Cs isotope ratios obtained by TIMS were more precise, by an order of magnitude, while the ICP-QQQ results possessed good abundance sensitivities. A slightly higher
Cs isotope ratios were measured, using both thermal ionization mass spectrometry (TIMS) and triple quadrupole induced coupled plasma mass spectrometry (ICP-QQQ). The results of each of these measurements were compared, and we found that Cs isotope ratios obtained by TIMS were more precise, by an order of magnitude, while the ICP-QQQ results possessed good abundance sensitivities. A slightly higher 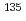 Cs/
Cs/ Cs ratio in the northwest area of the FDNPS was observed, while other areas exhibited similar values, all within the measurement error range, which indicated different origins of radiocesium. These results agreed with previously reported
Cs ratio in the northwest area of the FDNPS was observed, while other areas exhibited similar values, all within the measurement error range, which indicated different origins of radiocesium. These results agreed with previously reported 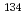 Cs/
Cs/ Cs activity distributions, suggesting that this ratio may be useful in identifying radiocesium origins for evaluating future nuclear reactor decommissions.
Cs activity distributions, suggesting that this ratio may be useful in identifying radiocesium origins for evaluating future nuclear reactor decommissions.
Tsukahara, Takehiko*; Saga, Kaname*; Suzuki, Hideya*; Matsumura, Tatsuro
Kurin Tekunoroji, 29(12), p.4 - 7, 2019/12
no abstracts in English
 -isopropylacrylamide) with diglycolamide-typed ligands
-isopropylacrylamide) with diglycolamide-typed ligandsSaga, Kaname*; Suzuki, Hideya; Matsumura, Tatsuro; Tsukahara, Takehiko*
Analytical Sciences, 35(4), p.461 - 464, 2019/04
Times Cited Count:4 Percentile:13.57(Chemistry, Analytical)The phase transition-based gelification phenomenon of poly- -isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
-isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
Tsukahara, Takehiko*; Suzuki, Hideya*; Matsumura, Tatsuro; Saga, Kaname*
Bunri Gijutsu, 49(4), p.221 - 225, 2019/04
no abstracts in English
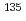 Cs/
Cs/ Cs isotope ratio near the Fukushima Daiichi Nuclear Power Station
Cs isotope ratio near the Fukushima Daiichi Nuclear Power StationShimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Shimada, Taro; Takeda, Seiji
no journal, ,
no abstracts in English
Ouchi, Kazuki; Muto, Yuki*; Brandt, A.*; Nabatame, Nozomi*; Tsukahara, Takehiko*; Kitatsuji, Yoshihiro
no journal, ,
The adsorption and elution performance of uranium with the microchip column (length 11 mm, column volume 0.39  L) loaded an anion exchange resin for faster and safer radioactive waste analysis were investigated. When a mixed sample of lanthanides and uranium was flown on the microchip column at the feed rate of 1 ml h
L) loaded an anion exchange resin for faster and safer radioactive waste analysis were investigated. When a mixed sample of lanthanides and uranium was flown on the microchip column at the feed rate of 1 ml h , uranium could be selectively adsorbed and eluted in about 4 minutes of operation time. This was faster than a conventional column which took several tens of minutes. When this column was applied to uranium separation of the standard seawater, the uranium concentration was determined to be 2.86
, uranium could be selectively adsorbed and eluted in about 4 minutes of operation time. This was faster than a conventional column which took several tens of minutes. When this column was applied to uranium separation of the standard seawater, the uranium concentration was determined to be 2.86 0.05 ppb, which was in good agreement with the certified value (2.81
0.05 ppb, which was in good agreement with the certified value (2.81 0.16 ppb). Therefore, the verification test was successful.
0.16 ppb). Therefore, the verification test was successful.
Arai, Yoichi; Watanabe, So; Hasegawa, Kenta; Okamura, Nobuo; Watanabe, Masayuki; Takeda, Keisuke*; Fukumoto, Hiroki*; Ago, Tomohiro*; Hagura, Naoto*; Tsukahara, Takehiko*
no journal, ,
Iwamoto, Toshihiro; Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
no journal, ,
The sludge generated production of nuclear fuel contained uranium has been storage. The sludge is immersed in some kinds of solution. After immersion, uranium is recovered from the solution. Cerium extractive tests using thermoresponsive polymer was carried out on two kinds of extractants. C14-BAMA was found to be superior, and we plan to conduct a uranium study on this extractant.
Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
no journal, ,
The sludge contained uranium generated production of nuclear fuel has been storage. The sludge is immersed in some kinds of solution. After immersion, uranium is recovered from the solution. Solvent extraction method, extraction chromatography and gelling extraction method were conducted on uranyl nitrate solution using monoamide extractant to compare on amount of waste and running cost on each methods. The gelling extraction method was superior to other two methods.
Shimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Takeda, Seiji
no journal, ,
Because of the accident at the Fukushima Daiichi Nuclear Power Station (1F), a large area of eastern Japan was contaminated by radiocesium. There are many old nuclear facilities in the area. When the old nuclear facilities will be decommissioned in three decades, the radioactive level of radiocesium from 1F would be more than clearance level. When radiocesium would be detected at the completion of their decommissioning in the site, estimation of the origin of which is from background originated 1F and fallout of atmospheric nuclear test or from decommissioned facilities will be important. Therefore, soils of 3 sites were collected by using U8 containers and by core boring, and  Cs radioactivity,
Cs radioactivity, 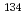 Cs/
Cs/ Cs radioactivity ratios,
Cs radioactivity ratios, 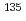 Cs/
Cs/ Cs isotope ratios were determined. Separation method of Cs from soil materials were developed based on the reported methods. The
Cs isotope ratios were determined. Separation method of Cs from soil materials were developed based on the reported methods. The  Cs radioactivity concentrations were varied in not only between sites but also in the same site, however,
Cs radioactivity concentrations were varied in not only between sites but also in the same site, however, 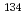 Cs/
Cs/ Cs radioactivity ratio was approximately constant in all sites. In case of highly contaminated soils,
Cs radioactivity ratio was approximately constant in all sites. In case of highly contaminated soils, 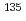 Cs/
Cs/ Cs isotope ratio was almost comparable to that for the soil collected at near the 1F. On the other hand, in case of lower contaminated soils,
Cs isotope ratio was almost comparable to that for the soil collected at near the 1F. On the other hand, in case of lower contaminated soils, 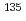 Cs/
Cs/ Cs isotope ratio was slightly higher than that for soil collected at near the 1F. It is possibly that fallout before the 1F accident contained
Cs isotope ratio was slightly higher than that for soil collected at near the 1F. It is possibly that fallout before the 1F accident contained 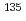 Cs to increase the
Cs to increase the 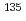 Cs/
Cs/ Cs isotope ratio. In the presentation, vertical distribution will also be discussed.
Cs isotope ratio. In the presentation, vertical distribution will also be discussed.
Kai, Masao; Iwamoto, Toshihiro; Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Nakamura, Masahiro; Tsukahara, Takehiko*; Itoda, Naokazu*; Naruse, Atsuki*
no journal, ,
The sludges containing uranium are generated in nuclear fuel fabrication process and have been stored in nuclear fuel fabrication facilities. Uranium is suggested to be selectively recovered from the solution in which the sludges are immersed. In this study, the oxide conversion tests were carried out with the gel obtained by the temperature swing extraction tests with cerium. The most effective heating temperature for the oxide conversion of was determined as 1000 degree Celsius. Based on the results of tests with cerium, the oxide conversion tests with uranium gel were also carried out. The gel was heated at 1000, and products were specified according to analysis data.
Sano, Yuichi; Watanabe, So; Takeuchi, Masayuki; Fukumoto, Hiroki*; Arai, Tsuyoshi*; Kim, S.*; Nakase, Masahiko*; Tsukahara, Takehiko*
no journal, ,
The research project on minor actinides recovery using extraction chromatography technology has been carried out for the reduction of volume and radiotoxicity of vitrified products. The overview of this project and main results are described in this paper.
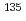 Cs/
Cs/ Cs isotope ratio
Cs isotope ratioShimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Kim, M. S.*; Shimada, Taro; Takeda, Seiji; Yamaguchi, Tetsuji
no journal, ,
Radiocesium from the Fukushima Daiichi Nuclear Power Station (1F) accident can be treated as background radioactivity at completion of decommissioning, so determining the origin of radiocesium is essential. Therefore, we studied identification of radiocesium origin using 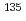 Cs/
Cs/ Cs isotope ratio. The ratio originated from 1F was 0.3628
Cs isotope ratio. The ratio originated from 1F was 0.3628 0.0005. When the ratio originated from decommissioning facility was 0.05
0.0005. When the ratio originated from decommissioning facility was 0.05 0.2 larger than that originated from 1F, it is challenging to decrease the stand deviation less than 0.01 for 1 Bq/g of
0.2 larger than that originated from 1F, it is challenging to decrease the stand deviation less than 0.01 for 1 Bq/g of  Cs because precision was decreased as decreasing the
Cs because precision was decreased as decreasing the  Cs concentration. If the ratio is able to be determined within the standard deviation of 0.0005 for 0.5 Bq/g of soil sample, the identification would be available.
Cs concentration. If the ratio is able to be determined within the standard deviation of 0.0005 for 0.5 Bq/g of soil sample, the identification would be available.
 Cs/
Cs/ Cs isotope ratio in environmental soil samples
Cs isotope ratio in environmental soil samplesShimada, Asako; Kim, M. S.*; Tsukahara, Takehiko*; Nomura, Masao*; Shimada, Taro; Takeda, Seiji; Yamaguchi, Tetsuji
no journal, ,
no abstracts in English
Tsukahara, Takehiko*; Brandt, A.*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki
no journal, ,
no abstracts in English