Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Matsumura, Daiju; Tsuji, Takuya; Kobayashi, Toru; Otobe, Haruyoshi; Kitatsuji, Yoshihiro
RSC Advances (Internet), 13(24), p.16321 - 16326, 2023/05
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)We clarified the chemical state transformation of deposits following the reduction of uranyl ion (U O
O
 ) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U
) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U is formed by the disproportionation of U
is formed by the disproportionation of U . (2) U
. (2) U forms U
forms U hydroxide deposits, and (3) finally, the hydroxide deposits transform into U
hydroxide deposits, and (3) finally, the hydroxide deposits transform into U oxide, generally having a larger electrical resistance than the former.
oxide, generally having a larger electrical resistance than the former.
Miyazaki, Tsukasa*; Miyata, Noboru*; Arima, Hiroshi*; Kira, Hiroshi*; Ouchi, Keiichi*; Kasai, Satoshi*; Tsumura, Yoshihiro*; Aoki, Hiroyuki
Langmuir, 37(32), p.9873 - 9882, 2021/08
Times Cited Count:5 Percentile:38.19(Chemistry, Multidisciplinary)Sekine, Yurina; Nankawa, Takuya; Yamada, Teppei*; Matsumura, Daiju; Nemoto, Yoshihiro*; Takeguchi, Masaki*; Sugita, Tsuyoshi; Shimoyama, Iwao; Kozai, Naofumi; Morooka, Satoshi
Journal of Environmental Chemical Engineering, 9(2), p.105114_1 - 105114_12, 2021/04
Times Cited Count:10 Percentile:49.28(Engineering, Environmental)Remediating toxic ion contamination is crucial for protecting human health and the environment. This study aimed to provide a powerful strategy for effectively utilizing bone waste from the food production and preparation industries for removal of toxic ions. Here, we show that immersing pig bone in NaHCO solution produced a carbonated nanohydroxyapatites (C-NHAP). The C-NHAP exhibited high adsorptivity for Sr
solution produced a carbonated nanohydroxyapatites (C-NHAP). The C-NHAP exhibited high adsorptivity for Sr , Cd
, Cd , Pb
, Pb , and Cu
, and Cu . The strontium adsorptivity was about 250 and 4,500 times higher than that of normal bone and synthetic HAP, respectively. The C-NHAP is an eco-friendly, high-performance material that is simple to prepare and should be useful for tackling problems of food waste disposal and environmental pollution.
. The strontium adsorptivity was about 250 and 4,500 times higher than that of normal bone and synthetic HAP, respectively. The C-NHAP is an eco-friendly, high-performance material that is simple to prepare and should be useful for tackling problems of food waste disposal and environmental pollution.
Hosoya, Yoshihiro*; Matsumura, Yuta*; Tomota, Yo*; Onuki, Yusuke*; Harjo, S.
ISIJ International, 60(9), p.2097 - 2106, 2020/09
Times Cited Count:5 Percentile:31.59(Metallurgy & Metallurgical Engineering)Miyazaki, Tsukasa*; Miyata, Noboru*; Yoshida, Tessei*; Arima, Hiroshi*; Tsumura, Yoshihiro*; Torikai, Naoya*; Aoki, Hiroyuki; Yamamoto, Katsuhiro*; Kanaya, Toshiji*; Kawaguchi, Daisuke*; et al.
Langmuir, 36(13), p.3415 - 3424, 2020/04
Times Cited Count:14 Percentile:58.64(Chemistry, Multidisciplinary)Hosoya, Yoshihiro*; Matsumura, Yuta*; Tomota, Yo*; Onuki, Yusuke*; Harjo, S.
Tetsu To Hagane, 106(3), p.154 - 164, 2020/03
Times Cited Count:2 Percentile:12.73(Metallurgy & Metallurgical Engineering)Miyazaki, Tsukasa*; Miyata, Noboru*; Asada, Mitsunori*; Tsumura, Yoshihiro*; Torikai, Naoya*; Aoki, Hiroyuki; Yamamoto, Katsuhiro*; Kanaya, Toshiji*; Kawaguchi, Daisuke*; Tanaka, Keiji*
Langmuir, 35(34), p.11099 - 11107, 2019/08
Times Cited Count:22 Percentile:67.64(Chemistry, Multidisciplinary) Zy
Zy N surrogate materials for fast reactor nitride fuel
N surrogate materials for fast reactor nitride fuelYoneda, Yasuhiro; Tsuji, Takuya; Matsumura, Daiju; Okamoto, Yoshihiro; Takaki, Seiya; Takano, Masahide
Transactions of the Materials Research Society of Japan, 42(2), p.23 - 26, 2017/04
ZnN is a possible candidate for the diluent material for nitride fuels containing transuranium elements. Pellets of inert matrix material ZrN, and surrogate nitride fuel material Dy Zr
Zr N, are fabricated for the purpose of investigating the crystal structure. Lattice parameters of Dy
N, are fabricated for the purpose of investigating the crystal structure. Lattice parameters of Dy Zr
Zr N followed the Vegard's low, in spite of the large lattice mismatch (
N followed the Vegard's low, in spite of the large lattice mismatch ( 7%) between DyN and ZrN. Local structure analysis was performed by X-ray absorption fine structure (XAFS) and atomic pair-distribution function (PDF) methods. The Zr-N nearest neighbor bond distance changed as changing the Dy composition. The complex local structure of DyN and ZrN is related to the preferable effects of ZrN.
7%) between DyN and ZrN. Local structure analysis was performed by X-ray absorption fine structure (XAFS) and atomic pair-distribution function (PDF) methods. The Zr-N nearest neighbor bond distance changed as changing the Dy composition. The complex local structure of DyN and ZrN is related to the preferable effects of ZrN.
 C
C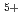 ion beam irradiation in chrysanthemum
ion beam irradiation in chrysanthemumMatsumura, Atsushi*; Nomizu, Toshikazu*; Furutani, Noriyuki*; Hayashi, Ken*; Minamiyama, Yasuhiro*; Hase, Yoshihiro
Scientia Horticulturae, 123(4), p.558 - 561, 2010/02
Times Cited Count:31 Percentile:79.03(Horticulture)Nakashima, Hiroshi; Sakamoto, Yukio; Iwamoto, Yosuke; Matsuda, Norihiro; Kasugai, Yoshimi; Nakane, Yoshihiro; Masukawa, Fumihiro; Mokhov, N.*; Leveling, A.*; Boehnlein, D.*; et al.
Nuclear Technology, 168(2), p.482 - 486, 2009/11
Times Cited Count:7 Percentile:45.28(Nuclear Science & Technology)Experimental studies of shielding and radiation effects have been started using 120-GeV proton synchrotron at Fermi National Accelerator Laboratory (FNAL) under collaboration between FNAL and Japan. The first campaign of the experiment was carried out at the Pbar target station and Numi experimental station at FNAL, using antiproton and neutrino production targets irradiated by 120-GeV protons. The generated secondary particles passing through steel, concrete and rock were measured by activation methods as well as by other detectors such as scintillator with a veto counter, phoswich detector and a Bonner ball counter on trial. Preliminary experimental results are presented.
Furutani, Noriyuki*; Matsumura, Atsushi*; Hase, Yoshihiro; Yoshihara, Ryohei; Narumi, Issei
JAEA-Review 2008-055, JAEA Takasaki Annual Report 2007, P. 69, 2008/11
no abstracts in English
Matsumura, Atsushi*; Furutani, Noriyuki*; Hase, Yoshihiro; Yokota, Yuichiro; Tanaka, Atsushi
JAEA-Review 2007-060, JAEA Takasaki Annual Report 2006, P. 78, 2008/03
no abstracts in English
 (
(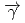 ,
,
 )
)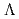 and
and  (
(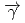 ,
,
 )
)
 reactions for
reactions for 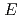
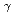 =1.5-2.4 GeV
=1.5-2.4 GeVZegers, R. G. T.*; Sumihama, Mizuki*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Dat , S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
Physical Review Letters, 91(9), p.092001_1 - 092001_4, 2003/08
Times Cited Count:128 Percentile:94.9(Physics, Multidisciplinary)no abstracts in English
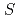 = +1 Baryon resonance in photoproduction from the neutron
= +1 Baryon resonance in photoproduction from the neutronNakano, Takashi*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Date, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; Fujiwara, Mamoru; et al.
Physical Review Letters, 91(1), p.012002_1 - 012002_4, 2003/07
Times Cited Count:1006 Percentile:99.86(Physics, Multidisciplinary)no abstracts in English
Asano, Yoshihiro; Matsumura, Toru; Chiba, R.*; Hashimoto, Tomoyuki*; Miura, Akio*; Shimizu, Hajime*; Tajima, Yasuhisa*; Yoshida, H.*
Nuclear Instruments and Methods in Physics Research A, 451(2), p.658 - 696, 2000/11
no abstracts in English
 " by means of synchrotron radiation
" by means of synchrotron radiationOno, Hirotaka*; Maeda, Nobuhiro*; Nakagawa, Sho*; Zushi, Yoshihiro*; Iwase, Akihiro*; Baba, Yuji; Hirao, Norie*; Ishikawa, Norito; Chimi, Yasuhiro; Sataka, Masao; et al.
no journal, ,
X-ray absorption spectroscopy is applied for evaluation of heavy ion irradiation effects in sim-fuel CeO in order to investigate the radiation damage of nuclear fuels bombarded by fission fragments. It is found that oxygen atoms are displaced via elastic displacement process, and the charge state of Ce changes from 4+ to 3+.
in order to investigate the radiation damage of nuclear fuels bombarded by fission fragments. It is found that oxygen atoms are displaced via elastic displacement process, and the charge state of Ce changes from 4+ to 3+.
Furutani, Noriyuki*; Matsumura, Atsushi*; Yoshihara, Ryohei; Hase, Yoshihiro
no journal, ,
no abstracts in English
Shiwaku, Hideaki; Yaita, Tsuyoshi; Suzuki, Shinichi; Kobayashi, Toru; Miyazaki, Yuji; Awual, M. R.; Motokawa, Ryuhei; Okamoto, Yoshihiro; Matsumura, Daiju; Yoshii, Kenji; et al.
no journal, ,
no abstracts in English
Shiwaku, Hideaki; Yaita, Tsuyoshi; Suzuki, Shinichi; Kobayashi, Toru; Miyazaki, Yuji; Awual, M. R.; Motokawa, Ryuhei; Okamoto, Yoshihiro; Matsumura, Daiju
no journal, ,
no abstracts in English
Saeki, Morihisa*; Yomogida, Takumi; Matsumura, Daiju; Saito, Takumi*; Okamoto, Yoshihiro; Oba, Hironori*
no journal, ,
The structures of isoploymolybdate(VI) were investigated by using laser Raman spectroscopy. The assignment of the Raman spectra showed presence of the [Mo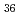 O
O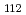 (H
(H O)
O)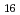 ]
]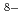 in the 0.2 M HNO
in the 0.2 M HNO and the [Mo
and the [Mo O
O (H
(H O)]
O)] in the 1.0 M HNO
in the 1.0 M HNO . To extract the spectrum of the intermediate species, we applied chemometrics analysis to a series of the observed spectra. The analysis revealed presence of one intermediate [Mo(VI)
. To extract the spectrum of the intermediate species, we applied chemometrics analysis to a series of the observed spectra. The analysis revealed presence of one intermediate [Mo(VI) O
O ]
]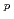 species in the 0.5 M HNO
species in the 0.5 M HNO . The intermediate species were assigned to [Mo
. The intermediate species were assigned to [Mo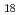 O
O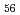 (H
(H O)
O) ]
] with the aid of X-ray absorption spectroscopy.
with the aid of X-ray absorption spectroscopy.