Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
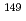 Sm M
Sm M ssbauer and Sm L
ssbauer and Sm L -edge X-ray absorption spectroscopies
-edge X-ray absorption spectroscopiesTsutsui, Satoshi; Higashinaka, Ryuji*; Nakamura, Raito*; Fujiwara, Kosuke*; Nakamura, Jin*; Kobayashi, Yoshio*; Ito, Takashi; Yoda, Yoshitaka*; Kato, Kazuo*; Nitta, Kiyofumi*; et al.
Hyperfine Interactions, 242(1), p.32_1 - 32_10, 2021/12
Times Cited Count:1 Percentile:84.42(Physics, Atomic, Molecular & Chemical)Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
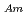 ) and Cm (
) and Cm (
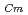 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:15 Percentile:52.81(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be  99% by optimizing separation conditions.
99% by optimizing separation conditions.
 -tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cell
-tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya; Hotoku, Shinobu; Kawasaki, Tomohiro*; Sagawa, Hiroshi*; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(1), p.27 - 37, 2019/00
Times Cited Count:21 Percentile:65.06(Chemistry, Multidisciplinary)A continuous counter-current experiment using TDdDGA was performed using mixer-settler extractors installed in a hot cell. Nitric acid containing minor actinides (MAs: Am and Cm), rare earths (REs: Y, La, Nd, and Eu), and other fission products (Sr, Cs, Zr, Mo, Ru, Rh, and Pd) was fed to the extractor. TDdDGA effectively extracted MAs and REs from the feed, while other fission products were barely extracted. The extracted MAs and REs were back-extracted by bringing them in contact with 0.02 mol/dm nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were
nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were  98% and
98% and  86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
 -site randomness on the antiferroelectric/relaxor nature of the ground state; Diffuse and inelastic X-ray scattering study of Pb(In
-site randomness on the antiferroelectric/relaxor nature of the ground state; Diffuse and inelastic X-ray scattering study of Pb(In Nb
Nb )O
)O
Owada, Kenji*; Tsukada, Shinya*; Fukuda, Tatsuo; Tsutsui, Satoshi*; Baron, A. Q. R.*; Mizuki, Junichiro*; Owa, Hidehiro*; Yasuda, Naohiko*; Terauchi, Hikaru*
Physical Review B, 98(5), p.054106_1 - 054106_10, 2018/08
Times Cited Count:3 Percentile:15.56(Materials Science, Multidisciplinary) ,
, -di(2-ethylhexyl)octanamide from nitric acid medium
-di(2-ethylhexyl)octanamide from nitric acid mediumTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 35(6), p.439 - 449, 2017/08
Times Cited Count:5 Percentile:16.24(Chemistry, Multidisciplinary)Solvent extraction of uranium from a nitric acid medium was performed with  ,
, -di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as
-di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as 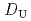 = 1.1
= 1.1![$$[rm NO^{-}_{3}]^{1.6}_{rm aq}[{rm DEHOA}]^{2}_{rm org}$$](/search/images/ERNVY2TNEBHE4XT1FV4V44ZTPVOV24ZRFY1H0X11LRZG0IDBOF4VW402OJWSARCFJBHUC5K3LZ3TE5K5PNOHE1JAN3ZGO5JE.gif) . Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation
. Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation 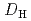 = 0.12
= 0.12![$$[rm H^{+}]^{0.76}_{rm aq}[{rm DEHOA_{rm Free}}]_{rm H}$$](/search/images/ERNVY2TNEBEF24ZLPVOV24ZQFY1TM5K5PNOHE1JAMFYX0W11LRZG0ICEIVEE4QK5PNOHE1JAIZZGKZL3PVOV4402OJWSASD3EQ.gif) was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm
was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm (M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
(M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
 -dialkylamides using multistage mixer-settler extractors
-dialkylamides using multistage mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Tsubata, Yasuhiro; Matsumura, Tatsuro
Procedia Chemistry, 21, p.156 - 161, 2016/12
Times Cited Count:5 Percentile:94.32(Chemistry, Inorganic & Nuclear)A continuous counter-current experiment was carried out to demonstrate the validity of a process using  -dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed
-dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed  -di(2-ethylhexyl)-2,2-dimethylpropanamide and
-di(2-ethylhexyl)-2,2-dimethylpropanamide and  -di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm
-di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm (M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5
(M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5 10
10 . These results supported the validity of the proposed process.
. These results supported the validity of the proposed process.
Futakawa, Masatoshi; Tsutsui, Kihei*; Kogawa, Hiroyuki; Naoe, Takashi
Key Engineering Materials, 715, p.203 - 209, 2016/11
The developments of the high power proton accelerators become a worldwide interest to provide various applications, where the targets are demanded to efficiently produce secondary beams and to survive intensive MW class proton beam power supplied by the accelerators. Solid metal targets might be melted by very high heat flux that is caused by the proton beam bombardment. In fact, the incident occurred at J-PARC, in which the gold solid target was locally melted to explosively jet molten gold. The jet collided with a structural beryllium flange plate that has a function of vacuum boundary. Some parts of gold were splashed and the other stuck on the flange plate. The relationship between the impact velocity and the morphology of the sticking pattern on the plate was quantitatively evaluated by introducing fractal analysis. It was found that the fractal dimension is correlated with the impact velocity and might be a useful factor to indicate the localized impact force and behavior.
Ban, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
A continuous counter-current experiment was conducted using mixer-settler extractors installed in a hot cell.  -Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) was used as the extractant for U. The experimental results showed that DEHDMPA can effectively extract U from nitric acid, and the recovery of U in the U fraction was obtained as 99.6%.
-Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) was used as the extractant for U. The experimental results showed that DEHDMPA can effectively extract U from nitric acid, and the recovery of U in the U fraction was obtained as 99.6%.
 ,
, -di(2-ethylhexyl)octanamide toward uranium from nitric acid
-di(2-ethylhexyl)octanamide toward uranium from nitric acidTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
Extraction properties of  ,
, -di(2-ethylhexyl)octanamide (DEHOA) toward uranium from nitric acid were studied using single-stage batch method. The results revealed that DEHOA effectively extracted uranium when the nitrate ion concentration was 2.0-3.0 mol/dm
-di(2-ethylhexyl)octanamide (DEHOA) toward uranium from nitric acid were studied using single-stage batch method. The results revealed that DEHOA effectively extracted uranium when the nitrate ion concentration was 2.0-3.0 mol/dm .
.
Suzuki, Asuka; Hotoku, Shinobu; Ban, Yasutoshi; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
The Japan Atomic Energy Agency (JAEA) conducts extensive research and development (R&D) activities toward the development of aqueous separation processes for spent nuclear fuel at the Nuclear Fuel Cycle Safety Engineering Research Facility-Back-end Fuel Cycle Key Element Research Facility (NUCEF-BECKY). BECKY is equipped with three hot cells, several glove boxes, and fume hoods. One of the hot cells, called the process cell, contains bench scale spent fuel test equipment used for U, Pu, and other spent fuel material. In this study, we describe an equipment used for aqueous separation processes in the process cell.
 ,
, -di(2-ethylhexyl)butanamide-nitric acid systems using turbidity measurements
-di(2-ethylhexyl)butanamide-nitric acid systems using turbidity measurementsTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Matsumura, Tatsuro
Separation Science and Technology, 51(6), p.961 - 967, 2016/02
Times Cited Count:1 Percentile:4.78(Chemistry, Multidisciplinary)Quantitative evaluation of the two-phase separation between  ,
, -di(2-ethylhexyl)butanamide (DEHBA) and tri-
-di(2-ethylhexyl)butanamide (DEHBA) and tri- -butyl phosphate (TBP) diluted with
-butyl phosphate (TBP) diluted with  -dodecane and uranyl nitrate solution in nitric acid medium was achieved using turbidity measurements. The turbidities of DEHBA were relatively high, particularly at high DEHBA concentrations, while that of TBP rapidly decreased irrespective of nitric acid concentration. A high concentration of DEHBA, nitric acid, and uranium increased the turbidities in the organic phase, which could be ascribed to the increase in viscosity. Distribution ratios of uranium were also measured, and it was indicated that turbidity did not have a critical effect on the distribution ratio when the turbidity was below a certain value.
-dodecane and uranyl nitrate solution in nitric acid medium was achieved using turbidity measurements. The turbidities of DEHBA were relatively high, particularly at high DEHBA concentrations, while that of TBP rapidly decreased irrespective of nitric acid concentration. A high concentration of DEHBA, nitric acid, and uranium increased the turbidities in the organic phase, which could be ascribed to the increase in viscosity. Distribution ratios of uranium were also measured, and it was indicated that turbidity did not have a critical effect on the distribution ratio when the turbidity was below a certain value.
 As
As using high-resolution inelastic X-ray scattering
using high-resolution inelastic X-ray scatteringMurai, Naoki*; Fukuda, Tatsuo; Kobayashi, Tatsuya*; Nakajima, Masamichi*; Uchiyama, Hiroshi*; Ishikawa, Daisuke*; Tsutsui, Satoshi*; Nakamura, Hiroki; Machida, Masahiko; Miyasaka, Shigeki*; et al.
Physical Review B, 93(2), p.020301_1 - 020301_5, 2016/01
Times Cited Count:7 Percentile:34.12(Materials Science, Multidisciplinary) -dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractors
-dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 34(1), p.37 - 47, 2016/01
Times Cited Count:9 Percentile:29.47(Chemistry, Multidisciplinary)The extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was
-di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was  3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
 -dialkylamides as extractants for U and Pu by continuous counter-current extractors
-dialkylamides as extractants for U and Pu by continuous counter-current extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Tsutsui, Nao; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1147 - 1152, 2015/09
Extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),  -di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.
-di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.
 ,
, -dialkylamides-nitric acid systems
-dialkylamides-nitric acid systemsTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Urabe, Shunichi; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1153 - 1157, 2015/09
 ,
, -Dialkylamides are promising alternative extractants to tri-
-Dialkylamides are promising alternative extractants to tri- -butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.
-butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.  ,
, -Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in
-Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in  -dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
-dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
 Zn
Zn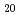 (T: Rh and Ir)
(T: Rh and Ir)Honda, Fuminori*; Hirose, Yusuke*; Miyake, Atsushi*; Mizumaki, Masaichiro*; Kawamura, Naomi*; Tsutsui, Satoshi*; Watanuki, Tetsu; Watanabe, Shinji*; Takeuchi, Tetsuya*; Settai, Rikio*; et al.
Journal of Physics; Conference Series, 592(1), p.012021_1 - 012021_5, 2015/03
Times Cited Count:3 Percentile:72.17(Physics, Atomic, Molecular & Chemical)no abstracts in English
 -di(2-
-di(2-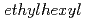 )-2,2-dimethylpropanamide (DEHDMPA) and
)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-
-di(2-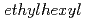 )butanamide (DEHBA) using mixer-settlers in the presence of degradation products of DEHDMPA and DEHBA
)butanamide (DEHBA) using mixer-settlers in the presence of degradation products of DEHDMPA and DEHBABan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction Research and Development, Japan, 22(1), p.47 - 55, 2015/00
Two sets of continuous counter-current experiments using mixer-setters were performed for evaluating extraction properties of  -dialkylamides toward U and Pu in the presence of their degradation products. The 1st cycle employed
-dialkylamides toward U and Pu in the presence of their degradation products. The 1st cycle employed  -di(2-
-di(2-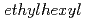 )-2,2-dimethylpropanamide (DEHDMPA) for selective extraction of U(VI), and the 2nd cycle employed
)-2,2-dimethylpropanamide (DEHDMPA) for selective extraction of U(VI), and the 2nd cycle employed  -di(2-
-di(2-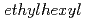 )butanamide (DEHDBA) for co-extraction of U(VI) and Pu(IV). Degradation products were added to the organic phase of each cycle. Most of U was effectively extracted by DEHDMPA, and the ratio of U recovered to the U fraction was 99.57%. DEHDMPA barely extracted Pu, and the decontamination factor of U with respect to Pu in the U fraction was 1.1
)butanamide (DEHDBA) for co-extraction of U(VI) and Pu(IV). Degradation products were added to the organic phase of each cycle. Most of U was effectively extracted by DEHDMPA, and the ratio of U recovered to the U fraction was 99.57%. DEHDMPA barely extracted Pu, and the decontamination factor of U with respect to Pu in the U fraction was 1.1  10
10 . The raffinate of the 1st cycle was used as the feed in the 2nd cycle, and the residual U and almost all Pu were effectively extracted by DEHBA. The degradation products had no detrimental effects on the two-phase separation and the operation of mixer-settlers.
. The raffinate of the 1st cycle was used as the feed in the 2nd cycle, and the residual U and almost all Pu were effectively extracted by DEHBA. The degradation products had no detrimental effects on the two-phase separation and the operation of mixer-settlers.
 nitric acid at elevated temperatures
nitric acid at elevated temperaturesBan, Yasutoshi; Hakamatsuka, Yasuyuki; Tsutsui, Nao; Urabe, Shunichi; Hagiya, Hiromichi; Matsumura, Tatsuro
Radiochimica Acta, 102(9), p.775 - 780, 2014/09
Times Cited Count:5 Percentile:36.96(Chemistry, Inorganic & Nuclear)Optical absorption spectra of Np in 3 mol/dm nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI),
nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI), 
 , were obtained at various temperature. The values of
, were obtained at various temperature. The values of 
 was found to decrease with increasing the temperature, and could be described by the equation
was found to decrease with increasing the temperature, and could be described by the equation 
 = -0.14
= -0.14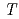 +85.5, where
+85.5, where 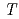 is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm
is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)]
nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)] /dt = 2.2
/dt = 2.2 10
10 exp[-65
exp[-65 10
10 /(
/(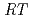 )][Np(V)]
)][Np(V)] , where
, where  and [Np(V)]
and [Np(V)] indicate the gas constant and Np(V) concentration at time
indicate the gas constant and Np(V) concentration at time 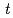 , respectively.
, respectively.
 Si
Si observed by X-ray absorption spectroscopy
observed by X-ray absorption spectroscopyNakai, Hirohito*; Ebihara, Takao*; Tsutsui, Satoshi*; Mizumaki, Masaichiro*; Kawamura, Naomi*; Michimura, Shinji*; Inami, Toshiya; Nakamura, Toshiyuki*; Kondo, Akihiro*; Kindo, Koichi*; et al.
Journal of the Physical Society of Japan, 82(12), p.124712_1 - 124712_5, 2013/12
Times Cited Count:9 Percentile:53.63(Physics, Multidisciplinary)The temperature and magnetic field dependences of Yb valence were observed in the heavy fermion compoundYbRh Si
Si by X-ray absorption spectroscopy. The measurements revealed that the Yb valence decreases with decreasing temperature in the range from 200 to 2 K and increases with increasing magnetic field in the range from 0 to 33 T without showing an abrupt change in the Yb valence. The Yb valence is in the range from 2.92 to 2.96 depending on temperature and magnetic field. With respect to the valence being 2.92 at 0 T and 2.93 at 33 T in 2 K, YbRh
by X-ray absorption spectroscopy. The measurements revealed that the Yb valence decreases with decreasing temperature in the range from 200 to 2 K and increases with increasing magnetic field in the range from 0 to 33 T without showing an abrupt change in the Yb valence. The Yb valence is in the range from 2.92 to 2.96 depending on temperature and magnetic field. With respect to the valence being 2.92 at 0 T and 2.93 at 33 T in 2 K, YbRh Si
Si is a valence fluctuation compound and does not reach the integer trivalent state at high magnetic field. These results endorse the conventional knowledge that the valence of Yb is very close to the integer value of 3+, decreases with decreasing temperature, and becomes closer to 3+ with increasing magnetic field.
is a valence fluctuation compound and does not reach the integer trivalent state at high magnetic field. These results endorse the conventional knowledge that the valence of Yb is very close to the integer value of 3+, decreases with decreasing temperature, and becomes closer to 3+ with increasing magnetic field.