Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modeling
on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modelingSasaki, Takayuki*; Ueda, Kenyo*; Saito, Takumi; Aoyagi, Noboru; Kobayashi, Taishi*; Takagi, Ikuji*; Kimura, Takaumi; Tachi, Yukio
Journal of Nuclear Science and Technology, 53(4), p.592 - 601, 2016/04
Times Cited Count:15 Percentile:77.12(Nuclear Science & Technology)The influences of pH and the concentrations of Eu and NaNO
and NaNO on the sorption of Eu
on the sorption of Eu to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO
to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO , whereas the Kd strongly depended on pH at 1 M NaNO
, whereas the Kd strongly depended on pH at 1 M NaNO . A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu
. A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu
sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH)
sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH) on the surface.
on the surface.
 (aq)/NH
(aq)/NH

Kobayashi, Taishi*; Sasaki, Takayuki*; Ueda, Kenyo*; Kitamura, Akira
Materials Research Society Symposium Proceedings, Vol.1518, p.231 - 236, 2013/10
For the nuclear waste management of TRU waste, it is necessary to assess the impact of nitrate salts contained in the waste. In the present study, the sorption behavior of Ni and Pd on the pumice tuff was investigated in the presence of NH (aq)/NH
(aq)/NH
 . Under different NH
. Under different NH (aq)/NH
(aq)/NH
 concentration, pH and ionic strength conditions, distribution coefficient (
concentration, pH and ionic strength conditions, distribution coefficient ( ) of Ni and Pd on the pumice tuff was determined by batch experiment. For Ni system, the
) of Ni and Pd on the pumice tuff was determined by batch experiment. For Ni system, the  values showed no significant dependence on the initial NH
values showed no significant dependence on the initial NH
 concentration in neutral pH region, agrees with the prediction from thermodynamic data. For Pd system, the
concentration in neutral pH region, agrees with the prediction from thermodynamic data. For Pd system, the  values decreased with an increase of [NH
values decreased with an increase of [NH
 ]ini, suggesting the formation of stable ammine complex (Pd(NH
]ini, suggesting the formation of stable ammine complex (Pd(NH )
)
 (
(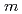 : 1 - 4)). The obtained
: 1 - 4)). The obtained  values for Ni and Pd were analyzed using the surface complexation model. By taking complexes predicted by thermodynamic data into account, the sorption behavior of Ni and Pd in the presence of NH
values for Ni and Pd were analyzed using the surface complexation model. By taking complexes predicted by thermodynamic data into account, the sorption behavior of Ni and Pd in the presence of NH (aq)/NH
(aq)/NH
 were well explained.
were well explained.
Ueda, Kenyo*; Sasaki, Takayuki*; Saito, Takumi*; Aoyagi, Noboru; Kimura, Takaumi; Tachi, Yukio
no journal, ,
no abstracts in English
Ueda, Kenyo*; Sasaki, Takayuki*; Saito, Takumi*; Aoyagi, Noboru; Kimura, Takaumi; Tachi, Yukio
no journal, ,
no abstracts in English
Ishii, Yasuo; Tachi, Yukio; Yoshikawa, Hideki; Sasaki, Takayuki*; Ueda, Kenyo*
no journal, ,
In order to investigate the sorption mechanisms of trivalent europium (Eu(III)) complexes at the illite surface, the sorption experiment of Eu(III) was performed in 0.1 M NaCl in the absence and presence of dissolved carbonate and in 0.1 M NaHCO solution by batch method. Kd value of Eu(III) in the absence of carbonate increased with increasing pH. On the other hand, in the cases where carbonate was present, there was its effect on Eu sorption. In the presence of dissolved carbonate, Kd values didn't increased in a higher pH region. In 0.1 M NaHCO
solution by batch method. Kd value of Eu(III) in the absence of carbonate increased with increasing pH. On the other hand, in the cases where carbonate was present, there was its effect on Eu sorption. In the presence of dissolved carbonate, Kd values didn't increased in a higher pH region. In 0.1 M NaHCO solution, Kd values decreased with increasing pH at pH
solution, Kd values decreased with increasing pH at pH 8, caused by the change of carbonate complexation of Eu(III). In the symposium, we will discuss a future subjects including mechanistic modeling and spectroscopic evaluation of surface chemical species based on a TRLFS experiment.
8, caused by the change of carbonate complexation of Eu(III). In the symposium, we will discuss a future subjects including mechanistic modeling and spectroscopic evaluation of surface chemical species based on a TRLFS experiment.
 on montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modeling
on montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modelingSasaki, Takayuki*; Ueda, Kenyo*; Saito, Takumi*; Aoyagi, Noboru; Kobayashi, Taishi*; Takagi, Ikuji*; Kimura, Takaumi; Tachi, Yukio
no journal, ,
The influence of pH, ionic strength, and the concentrations of Eu and nitrate salts on the sorption of Eu
and nitrate salts on the sorption of Eu on Na-montmorillonite was investigated by batch sorption measurements. The distribution coefficient (Kd) indicated that the cation exchange reaction is significant in pH 4-7 at low ionic strength, and the formation of surface complexation is related to the pH dependence of Kd at high ionic strength. The Eu
on Na-montmorillonite was investigated by batch sorption measurements. The distribution coefficient (Kd) indicated that the cation exchange reaction is significant in pH 4-7 at low ionic strength, and the formation of surface complexation is related to the pH dependence of Kd at high ionic strength. The Eu surface species were investigated using time-resolved laser fluorescence spectroscopy (TRLFS) with parallel factor analysis (PARAFAC). The PARAFAC modeling indicated two surface species, the outer-sphere (factor A) and inner-sphere (factor B) Eu
surface species were investigated using time-resolved laser fluorescence spectroscopy (TRLFS) with parallel factor analysis (PARAFAC). The PARAFAC modeling indicated two surface species, the outer-sphere (factor A) and inner-sphere (factor B) Eu complexes. Factor B was dominant at high pH and ionic strength, which was determined to be a Eu colloidal species formed at high Eu
complexes. Factor B was dominant at high pH and ionic strength, which was determined to be a Eu colloidal species formed at high Eu concentrations. A cation exchange model combined with a one-site non-electrostatic surface complexation model was applied to the measured Kd data, using the results of the TRLFS-PARAFAC analyses.
concentrations. A cation exchange model combined with a one-site non-electrostatic surface complexation model was applied to the measured Kd data, using the results of the TRLFS-PARAFAC analyses.