Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Jarrige, I.*; Ishii, Kenji; Matsumura, Daiju; Nishihata, Yasuo; Yoshida, Masahiro*; Kishi, Hirofumi*; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*; Kasai, Hideaki*; et al.
ACS Catalysis, 5(2), p.1112 - 1118, 2015/02
Times Cited Count:23 Percentile:46.16(Chemistry, Physical) O(111) surfaces; The Case of NO dissociation
O(111) surfaces; The Case of NO dissociationKishi, Hirofumi*; Padama, A. A. B.*; Arevalo, R. L.*; Moreno, J. L. V.*; Kasai, Hideaki*; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*; Nishihata, Yasuo
Journal of Physics; Condensed Matter, 24(26), p.262001_1 - 262001_5, 2012/07
Times Cited Count:9 Percentile:36.61(Physics, Condensed Matter)no abstracts in English
 O(111) surfaces; A Density functional theory based study
O(111) surfaces; A Density functional theory based studyPadama, A. A. B.*; Kishi, Hirofumi*; Arevalo, R. L.*; Moreno, J. L. V.*; Kasai, Hideaki*; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*; Nishihata, Yasuo
Journal of Physics; Condensed Matter, 24(17), p.175005_1 - 175005_6, 2012/05
Times Cited Count:40 Percentile:78.20(Physics, Condensed Matter)no abstracts in English
Matsumura, Daiju; Nishihata, Yasuo; Mizuki, Junichiro; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*
Journal of Applied Physics, 107(12), p.124319_1 - 124319_5, 2010/06
Times Cited Count:21 Percentile:58.65(Physics, Applied)The structural transformation of a Pd-perovskite automotive catalyst, LaFe Pd
Pd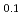 O
O , which has a high catalytic activity during aging, was studied by in situ time-resolved dispersive X-ray absorption fine structure spectroscopy at 473-773 K. An Al
, which has a high catalytic activity during aging, was studied by in situ time-resolved dispersive X-ray absorption fine structure spectroscopy at 473-773 K. An Al O
O -based conventional catalyst was also studied. In a reductive atmosphere, both catalysts showed similar temperature dependences of structural transformation from an oxide to a metal. However, different temperature dependence was observed in an oxidative atmosphere. A faster response in the structural change was observed in the Pd-perovskite catalyst than in the Pd/Al
-based conventional catalyst was also studied. In a reductive atmosphere, both catalysts showed similar temperature dependences of structural transformation from an oxide to a metal. However, different temperature dependence was observed in an oxidative atmosphere. A faster response in the structural change was observed in the Pd-perovskite catalyst than in the Pd/Al O
O catalyst. It was revealed that Pd-perovskite shows a considerably fast structural change to the oxidized state via the movement of Pd atoms into the perovskite crystal, in comparison with Pd/Al
catalyst. It was revealed that Pd-perovskite shows a considerably fast structural change to the oxidized state via the movement of Pd atoms into the perovskite crystal, in comparison with Pd/Al O
O showing two-step structural change for making PdO.
showing two-step structural change for making PdO.
 under redox atmosphere and CO/NO catalytic reaction studied by dispersive XAFS
under redox atmosphere and CO/NO catalytic reaction studied by dispersive XAFSMatsumura, Daiju; Okajima, Yuka; Nishihata, Yasuo; Mizuki, Junichiro; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*
Journal of Physics; Conference Series, 190, p.012154_1 - 012154_6, 2009/11
Times Cited Count:11 Percentile:90.30(Physics, Condensed Matter)Pd/LaFeO is known to keep the metal particle size small even after the long time redox processes because Pd atoms make complex oxide with LaFeO
is known to keep the metal particle size small even after the long time redox processes because Pd atoms make complex oxide with LaFeO perovskite-type crystal under the oxidative atmosphere. We observed the local structure of Pd atoms by dispersive optics from the viewpoint of dynamical structure change of Pd during metal-oxide change and CO-NO catalytic reaction. It was recognized that, under the reductive atmosphere, Pd atoms show similar speed of movement from oxide to metal state both on LaFeO
perovskite-type crystal under the oxidative atmosphere. We observed the local structure of Pd atoms by dispersive optics from the viewpoint of dynamical structure change of Pd during metal-oxide change and CO-NO catalytic reaction. It was recognized that, under the reductive atmosphere, Pd atoms show similar speed of movement from oxide to metal state both on LaFeO and Al
and Al O
O . However, under the oxidative atmosphere, Pd atoms on LaFeO
. However, under the oxidative atmosphere, Pd atoms on LaFeO show faster movement from metal to oxide state with single reaction step than those on Al
show faster movement from metal to oxide state with single reaction step than those on Al O
O with two-step oxidation. In addition, many differences in structure and shape between Pd particles on LaFeO
with two-step oxidation. In addition, many differences in structure and shape between Pd particles on LaFeO and Al
and Al O
O are observed.
are observed.
 perovskite automotive catalyst by high-speed analysis with in situ DXAFS
perovskite automotive catalyst by high-speed analysis with in situ DXAFSUenishi, Mari*; Tanaka, Hirohisa*; Taniguchi, Masashi*; Tan, Isao*; Nishihata, Yasuo; Mizuki, Junichiro; Kobayashi, Tetsuhiko*
Catalysis Communications, 9(2), p.311 - 314, 2008/02
Times Cited Count:33 Percentile:61.52(Chemistry, Physical)In the LaFePdO perovskite catalyst, redox fluctuations of the exhaust gas suppress the growth of particles of precious metal by causing Pd to move in and out of the perovskite crystal. To observe the real movements of Pd directly, the time evolution of local structure around Pd by redox fluctuations was investigated by in situ energy-dispersive X-ray absorption fine-structure (DXAFS) analysis with a 10ms resolution. We proved that the change in structure of Pd is sufficiently fast to respond to the control frequency (1-4Hz) of an actual gasoline engine and that the Pd particles that segregate out are extremely fine.
perovskite catalyst, redox fluctuations of the exhaust gas suppress the growth of particles of precious metal by causing Pd to move in and out of the perovskite crystal. To observe the real movements of Pd directly, the time evolution of local structure around Pd by redox fluctuations was investigated by in situ energy-dispersive X-ray absorption fine-structure (DXAFS) analysis with a 10ms resolution. We proved that the change in structure of Pd is sufficiently fast to respond to the control frequency (1-4Hz) of an actual gasoline engine and that the Pd particles that segregate out are extremely fine.
Tanaka, Hirohisa*; Taniguchi, Masashi*; Uenishi, Mari*; Kajita, Nobuhiko*; Tan, Isao*; Nishihata, Yasuo; Mizuki, Junichiro; Narita, Keiichi*; Kimura, Mareo*; Kaneko, Kimiyoshi*
Angewandte Chemie; International Edition, 45(36), p.5998 - 6002, 2006/09
Times Cited Count:203 Percentile:95.40(Chemistry, Multidisciplinary)no abstracts in English
Tanaka, Hirohisa*; Uenishi, Mari*; Taniguchi, Masashi*; Tan, Isao*; Narita, Keiichi*; Kimura, Mareo*; Kaneko, Kimiyoshi*; Nishihata, Yasuo; Mizuki, Junichiro
Catalysis Today, 117(1-3), p.321 - 328, 2006/09
Times Cited Count:211 Percentile:98.04(Chemistry, Applied)no abstracts in English
Tanaka, Hirohisa*; Tan, Isao*; Uenishi, Mari*; Taniguchi, Masashi*; Nishihata, Yasuo; Mizuki, Junichiro
Key Engineering Materials, 317-318, p.827 - 832, 2006/08
no abstracts in English
Tan, Isao*; Taniguchi, Masashi*; Tanaka, Hirohisa*; Uenishi, Mari*; Kajita, Nobuhiko*; Nishihata, Yasuo; Mizuki, Junichiro; Niihara, Koichi*
Key Engineering Materials, 317-318, p.833 - 836, 2006/08
no abstracts in English
 perovskite automotive catalyst having a self-regenerative function
perovskite automotive catalyst having a self-regenerative functionTanaka, Hirohisa*; Tan, Isao*; Uenishi, Mari*; Taniguchi, Masashi*; Kimura, Mareo*; Nishihata, Yasuo; Mizuki, Junichiro
Journal of Alloys and Compounds, 408-412, p.1071 - 1077, 2006/02
Times Cited Count:58 Percentile:89.61(Chemistry, Physical)no abstracts in English
Naito, Kazuya*; Tanaka, Hirohisa*; Taniguchi, Masashi*; Uenishi, Mari*; Tan, Isao*; Kajita, Nobuhiko*; Takahashi, Ichiro*; Suzuki, Hiromasa*; Narita, Keiichi*; Hirai, Akimasa*; et al.
SAE 2006 World Congress & Exhibition Technical Papers, 8 Pages, 2006/00
no abstracts in English
Nishihata, Yasuo; Mizuki, Junichiro; Akao, Takahiro; Tanaka, Hirohisa*; Uenishi, Mari*; Kimura, Mareo*; Okamoto, Tokuhiko*; Hamada, Noriaki*
Nature, 418(6894), p.164 - 167, 2002/07
Times Cited Count:1030 Percentile:99.82(Multidisciplinary Sciences)no abstracts in English
Tanaka, Hirohisa*; Uenishi, Mari*; Tan, Isao*; Kimura, Mareo*; Mizuki, Junichiro; Nishihata, Yasuo
SAE 2001 World Congress Paper; Advanced Catalytic Converters and Substrates for Gasoline Emission Systems (SP1573), p.1 - 8, 2001/00
The catalyst of the crystalline ceramics known as a perovskite-type oxide was designed and controlled at the atomic level in order to create a new function for self-regeneration of precious metals in a usage ambience without auxiliary treatment.
Nishihata, Yasuo; Matsumura, Daiju; Okajima, Yuka; Mizuki, Junichiro; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*
no journal, ,
no abstracts in English
Taniguchi, Masashi*; Kan, C. Y.*; Uenishi, Mari*; Tanaka, Hirohisa*; Matsumura, Daiju; Nishihata, Yasuo; Mizuki, Junichiro; Uozumi, Akifumi*; Hamada, Ikutaro*; Morikawa, Yoshitada*
no journal, ,
no abstracts in English
Matsumura, Daiju; Okajima, Yuka; Nishihata, Yasuo; Mizuki, Junichiro; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*
no journal, ,
Dynamic structure changes of Pd metal particles on LaFeO and Al
and Al O
O were observed by dispersive XAFS optics during successive gas change of CO/NO. Observation rate of 0.2 Hz for dispersive XAFS enabled us to study precise structure of Pd metal particles under oxidation-reduction, expansion-contraction and sintering-dispersion transformations during gas change. Especially, it was revealed that the Pd particles on Al
were observed by dispersive XAFS optics during successive gas change of CO/NO. Observation rate of 0.2 Hz for dispersive XAFS enabled us to study precise structure of Pd metal particles under oxidation-reduction, expansion-contraction and sintering-dispersion transformations during gas change. Especially, it was revealed that the Pd particles on Al O
O show faster reaction for expansion of lattice under CO atmosphere and inner oxidation under NO atmosphere than those on LaFeO
show faster reaction for expansion of lattice under CO atmosphere and inner oxidation under NO atmosphere than those on LaFeO . Depending on the interaction between metal particle and support, many surface adsorption structure on Pd metal particles were influenced.
. Depending on the interaction between metal particle and support, many surface adsorption structure on Pd metal particles were influenced.
Matsumura, Daiju; Okajima, Yuka; Nishihata, Yasuo; Mizuki, Junichiro; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*
no journal, ,
Dynamic structure changes of Pd metal particles on LaFeO and Al
and Al O
O were observed by dispersive XAFS optics during successive gas change of CO/NO. Observation rate of 0.2 Hz for dispersive XAFS enabled us to study precise structure of Pd metal particles under oxidation-reduction, expansion-contraction and sintering-dispersion transformations during gas change. Especially, it was revealed that the Pd particles on Al
were observed by dispersive XAFS optics during successive gas change of CO/NO. Observation rate of 0.2 Hz for dispersive XAFS enabled us to study precise structure of Pd metal particles under oxidation-reduction, expansion-contraction and sintering-dispersion transformations during gas change. Especially, it was revealed that the Pd particles on Al O
O show faster reaction for expansion of lattice under CO atmosphere and inner oxidation under NO atmosphere than those on LaFeO
show faster reaction for expansion of lattice under CO atmosphere and inner oxidation under NO atmosphere than those on LaFeO . High relative precision study showed many undetected changes of Pd metal particles.
. High relative precision study showed many undetected changes of Pd metal particles.
Matsumura, Daiju; Okajima, Yuka; Nishihata, Yasuo; Mizuki, Junichiro; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*
no journal, ,
Metal particles used in the catalytic reaction are made fine in order to spread the surface area. However, it is expected that the nanometer-sized metal particles show the original catalytic reaction which is different from that for the larger particles. In order to study the originality of the fine particles, detailed structure, shape and electronic informations will be required. Dispersive XAFS system enables us to observe the fine particles with high precise information. We used this method for the study of the changes in the Pd metal fine particles during CO/NO catalytic reaction. As a result, the expansion-contraction, aggregation-dispersion and oxidation-reduction changes in the Pd metal fine particles have been revealed by direct observation of dispersive XAFS method.
Matsumura, Daiju; Nishihata, Yasuo; Mizuki, Junichiro; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*
no journal, ,
In-situ and real-time structural transformations of perovskite-type automotive catalysts having a high catalytic activity during aging were observed by dispersive XAFS method. Alumina-based conventional catalysts were also observed for reference. The experiments were operated at the beamline of SPring-8 BL14B1. Just after dosing by oxygen or hydrogen, continuous XAFS spectra were measured at 20-50 Hz in order to reveal the local structural transformation of precious metals. For the Pd-perovskite catalyst, the slower response of structural transformation was observed than the alumina-based catalyst under the oxygen atmosphere. Similar results were observed in Pt-based catalyst. On the other hand, there were no clear differences between the perovskite-type and alumina-based catalyst of Rh.