Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Aoyama, Takahito; Ueno, Fumiyoshi; Sato, Tomonori; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Otani, Kyohei; Igarashi, Takahiro
Annals of Nuclear Energy, 214, p.111229_1 - 111229_6, 2025/05
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Cantarel, V.; Chupin, F.; Ortega-Charlot, M.*; Yamagishi, Isao; Ueno, Fumiyoshi
Journal of Nuclear Materials, 592, p.154969_1 - 154969_9, 2024/04
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Maeda, Masaki*; Tanabe, Tadao*; Nishiwaki, Tomoya*; Aoki, Takayuki*; Dozaki, Koji*; Nishimura, Koshiro*; Fujii, Sho*; Ueno, Fumiyoshi; Tanaka, Akio*; Suzuki, Yusuke*; et al.
Transactions of the 27th International Conference on Structural Mechanics in Reactor Technology (SMiRT 27) (Internet), 10 Pages, 2024/03
 Sr and
Sr and  Cs in HCl solutions on the corrosion potential of Type 316L stainless steel
Cs in HCl solutions on the corrosion potential of Type 316L stainless steelAoyama, Takahito; Sato, Tomonori; Ueno, Fumiyoshi; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Igarashi, Takahiro
Zairyo To Kankyo, 72(11), p.284 - 288, 2023/11
no abstracts in English
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Corrosion, 78(6), p.503 - 515, 2022/06
Times Cited Count:1 Percentile:7.57(Materials Science, Multidisciplinary)The effects of electrochemical potential (ECP) on water chemistry within a crevice are of critical importance for understanding stress corrosion cracking (SCC) of Fe-Cr-Ni alloys in high temperature water. In this study, the effects of ECP on the electrical conductivity of a solution within a Type-316L stainless steel crevice (
 ) have been studied in 288
) have been studied in 288 C and 8 MPa water containing 10 ppb Cl
C and 8 MPa water containing 10 ppb Cl as major anionic species. In situ measurements of
as major anionic species. In situ measurements of 
 in a rectangular crevice with a gap of 15
in a rectangular crevice with a gap of 15  m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in
m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in 
 from 12
from 12  Scm
Scm to 160
to 160  Scm
Scm at a distance of 21 mm from the crevice mouth. The increase in
at a distance of 21 mm from the crevice mouth. The increase in 
 reached a maximum at about 0.15 V (about 300
reached a maximum at about 0.15 V (about 300  Scm
Scm ) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl
) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl is the major anionic species transported into the crevice at relatively low potentials, and that
is the major anionic species transported into the crevice at relatively low potentials, and that 
 increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
 generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(5), p.133 - 137, 2022/05
The effect of the corrosion inhibitor on the corrosion of steel under a thin solution layer was investigated. As a result of forming a thin solution layer with a thickness of 1.0-0.2 mm on the specimen, adding a mixed solution of sodium molybdate and aluminum lactate as a corrosion inhibitor, and performing electrochemical measurement, the corrosion inhibitor suppresses the anodic reaction. And in the thin solution layer, it was suggested that the morphology of the protective layer structure by the corrosion inhibitor changed according to the amount of liquid as compared with the bulk immersion.
Ishijima, Yasuhiro; Yokoyama, Kenichi*; Ueno, Fumiyoshi; Abe, Hitoshi
Materials Transactions, 63(4), p.592 - 599, 2022/04
Times Cited Count:2 Percentile:15.54(Materials Science, Multidisciplinary)The effect of thermal aging on the behavior of vacancy-hydrogen (H) cluster generation and the mechanical properties of tantalum (Ta) were investigated by positron annihilation lifetime spectroscopy (PALS) and tensile tests. Based on the PALS results, vacancy clusters that included 8-15 vacancies were generated after aging at and above 200 C, and vacancy-H clusters were generated in 3 and 6 h-charged specimens after aging at 300
C, and vacancy-H clusters were generated in 3 and 6 h-charged specimens after aging at 300 C. The loss of ductility in Ta and crack generation were observed by conducting tensile tests in 6 h H-charged specimens after aging at 200
C. The loss of ductility in Ta and crack generation were observed by conducting tensile tests in 6 h H-charged specimens after aging at 200 C for 2000 h and in 3 and 6 h H-charged specimens aged at 300
C for 2000 h and in 3 and 6 h H-charged specimens aged at 300 C for 2000 h. The reduction of ductility due to thermal aging of Ta was also observed under these thermal aging conditions. These results suggested that vacancy-H cluster generated by dissolved H during thermal aging induced the ductility loss by reducing the dislocation migration during deformation.
C for 2000 h. The reduction of ductility due to thermal aging of Ta was also observed under these thermal aging conditions. These results suggested that vacancy-H cluster generated by dissolved H during thermal aging induced the ductility loss by reducing the dislocation migration during deformation.
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Materials Transactions, 63(4), p.538 - 544, 2022/04
Times Cited Count:2 Percentile:15.54(Materials Science, Multidisciplinary)The time dependence of the corrosion behavior of tantalum (Ta), which is used in nuclear fuel reprocessing equipment, in sodium hydroxide (NaOH) solutions was investigated by immersion tests, and the mechanism of the time dependence was examined via surface observations and electrochemical measurements. The immersion tests were conducted at room temperature with NaOH concentrations ranging from 1 to 7 mol/L for immersion periods of 24 to 168 h. The corrosion rate increased with the NaOH concentration but peaked with the immersion period and then decreased. The time to peak of the corrosion rate was shorter with higher NaOH concentration. The X-ray diffraction (XRD) patterns and Raman spectra of the surfaces of the specimens immersed in the 7 mol/L NaOH solution for more than 48 h showed Na Ta
Ta O
O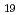 formation. The polarization resistance decreased with immersion time for all NaOH concentrations up to about 24 h after immersion. Thereafter, the polarization resistance increased with immersion time in 7 mol/L NaOH solution and remained almost constant in the other NaOH concentrations. Findings suggested that the change in the corrosion rate was affected by the film formation during immersion, since the time dependence of the polarization resistance and the sum of film resistance and charge transfer resistance had the same tendencies. The precipitation film was mainly Na
formation. The polarization resistance decreased with immersion time for all NaOH concentrations up to about 24 h after immersion. Thereafter, the polarization resistance increased with immersion time in 7 mol/L NaOH solution and remained almost constant in the other NaOH concentrations. Findings suggested that the change in the corrosion rate was affected by the film formation during immersion, since the time dependence of the polarization resistance and the sum of film resistance and charge transfer resistance had the same tendencies. The precipitation film was mainly Na Ta
Ta O
O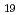 formed by the dissolution of the passivity film on Ta.
formed by the dissolution of the passivity film on Ta.
 Sr dissolved solution on corrosion potential of type 316L stainless steel
Sr dissolved solution on corrosion potential of type 316L stainless steelAoyama, Takahito; Kato, Chiaki; Sato, Tomonori; Sano, Naruto; Yamashita, Naoki; Ueno, Fumiyoshi
Zairyo To Kankyo, 71(4), p.110 - 115, 2022/04
no abstracts in English
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(4), p.121 - 125, 2022/04
The effect of solution layer thickness on the atmospheric corrosion of carbon steel was investigated using novel devices fabricated by a 3D printer. These novel devices allowed us to control the solution layer thickness precisely. Potentiodynamic polarization measurements were performed under thickness-controlled solution layer, and oxygen diffusion limiting current density ( ) and anodic current density (
) and anodic current density ( ) were measured. As the solution layer become thinner,
) were measured. As the solution layer become thinner,  increased and
increased and  decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20
decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20 10
10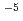 cm
cm s
s from the relationship between
from the relationship between  and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
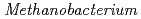 strain induces severe corrosion by retrieving electrons from Fe
strain induces severe corrosion by retrieving electrons from Fe under a freshwater environment
under a freshwater environmentHirano, Shinichi*; Ihara, Sota*; Wakai, Satoshi*; Dotsuta, Yuma; Otani, Kyohei; Kitagaki, Toru; Ueno, Fumiyoshi; Okamoto, Akihiro*
Microorganisms (Internet), 10(2), p.270_1 - 270_12, 2022/02
Times Cited Count:14 Percentile:81.48(Microbiology)To understand the role of methanogens in corrosion under anoxic conditions in freshwater, we investigated the corrosion activities of methanogens in samples collected from groundwater and rivers. We enriched microorganisms that can grow with CO /NaHCO
/NaHCO and Fe
and Fe as the sole carbon source and electron donor, respectively, in ground fresh water. Electrochemical analysis revealed that
as the sole carbon source and electron donor, respectively, in ground fresh water. Electrochemical analysis revealed that 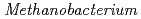 strain can uptake electrons from the cathode at lower than -0.61 V vs SHE and has a redox-active component with electrochemical potential different from those of other previously reported methanogens with extracellular electron transfer ability. This study indicated the corrosion risk by methanogens capable of taking up electrons from Fe
strain can uptake electrons from the cathode at lower than -0.61 V vs SHE and has a redox-active component with electrochemical potential different from those of other previously reported methanogens with extracellular electron transfer ability. This study indicated the corrosion risk by methanogens capable of taking up electrons from Fe in anoxic freshwater environments and the necessity of understanding the corrosion mechanism to contribute to risk diagnosis.
in anoxic freshwater environments and the necessity of understanding the corrosion mechanism to contribute to risk diagnosis.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 71(2), p.40 - 45, 2022/02
The purpose of this study is to investigate the effect of oxygen concentration in the air on the corrosion rate of carbon steel in an air/solution alternating environment in the low oxygen concentration range and to clarify the corrosion rate and corrosion mechanism of carbon steel depending on the oxygen concentration in air by the mass change of specimens before and after the corrosion test and observing the iron rust layer formed on the surface of carbon steel. The corrosion rate increases with increasing oxygen concentration in the air, and the gradient of the corrosion rate decreases gradually. The maximum erosion depth increased with increasing oxygen concentration except for the case of 1% oxygen concentration, however, the maximum erosion depth for 1% oxygen concentration was larger than that for 5% air oxygen concentration.
Wakai, Satoshi*; Hirano, Shinichi*; Ueno, Fumiyoshi; Okamoto, Akihiro*
Zairyo To Kankyo, 70(12), p.491 - 496, 2021/12
After Fukushima Daiichi Nuclear Power Station accident, various corrosion mitigating activities have been treated, and severe corrosion incident have never taken placed. On the other hand, the facilities were exposed sea water, and some of them have continuously exposed to ground water. The exposure of metal materials to environmental water has a risk of microbiologically influenced corrosion (MIC). In this paper, we summarize the latest knowledge of MIC and the task of MIC in the decommissioning of Fukushima Daiichi Nuclear Power Station.
Sato, Tomonori; Hata, Kuniki; Kaji, Yoshiyuki; Ueno, Fumiyoshi; Inoue, Hiroyuki*; Taguchi, Mitsumasa*; Seito, Hajime*; Tada, Eiji*; Abe, Hiroshi*; Akiyama, Eiji*; et al.
JAEA-Review 2021-001, 123 Pages, 2021/06
In the implement of the decommissioning of Fukushima Daiichi Nuclear Power Station (1F), there are many problems to be solved. Specially, the mitigation of the aging degradation by the corrosion of the structural materials is important to implement the decommissioning safely and continuously. However, there are limited data for the environmental factors of corrosion in 1F, and the condition of 1F is continuously changing. So, the literature data for the water radiolysis and the corrosion under irradiation are listed as the database of corrosion under irradiation in this report. And the new obtained radiolysis and corrosion data, which have not been reported in the literature and will be required in the decommissioning of 1F, are reported.
Igarashi, Takahiro; Komatsu, Atsushi; Motooka, Takafumi*; Ueno, Fumiyoshi; Yamamoto, Masahiro
Corrosion Science and Technology, 20(3), p.105 - 111, 2021/06
We constructed three dimensional computational model using cellular automata method to simulate the intergranular corrosion propagation of stainless steel. In the model, the computational system was constructed by three types of cells: grain (bulk), grain boundary (GB), and solution cell. Our simulations revealed that the surface roughness calculated by the model adopted distributed dissolution rates of GBs was greater than that adopted constant dissolution rates of GBs. The cross-sectional images obtained by our simulation were comparable with that obtained by corrosion tests. These results indicate that the surface roughness during corrosion relates the distribution of corrosion rate.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi
Materials Transactions, 62(6), p.763 - 769, 2021/06
Times Cited Count:1 Percentile:4.99(Materials Science, Multidisciplinary)In this study, the iron rust layer formed on the low alloy steel under air-solution alternating conditions was investigated by cross-sectional observation and analysis, and the mechanism of the accelerated corrosion of the steel under alternating conditions was clarified. The observations and analysis showed that the multilayered iron rust layer composed of the red rust layer (FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on the low alloy steel. It can be considered that the multilayered iron rust layer accelerated the cathodic reaction rate of dissolved oxygen under alternating conditions. This acceleration is the reason why the corrosion rate of the low alloy steel under alternating conditions was accelerated.
), and inner rust layer was formed on the low alloy steel. It can be considered that the multilayered iron rust layer accelerated the cathodic reaction rate of dissolved oxygen under alternating conditions. This acceleration is the reason why the corrosion rate of the low alloy steel under alternating conditions was accelerated.
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Zairyo To Kankyo, 70(6), p.192 - 198, 2021/06
The time dependence of corrosion behavior on tantalum used in nuclear fuel reprocessing equipment in sodium hydroxide solution was investigated by immersion corrosion tests, and the mechanism of aging change was discussed from surface observations and electrochemical measurements. The immersion tests were carried out at room temperature with NaOH concentrations ranging from 1 to 7 mol/L and immersion times ranging from 24 to 168 hr, respectively. The corrosion rate increased with NaOH concentration, but peaked with immersion time and then decreased. The time to peak of corrosion rate was shorter with higher NaOH concentration. The SEM observations and Raman analysis at the surface of the specimens that were cleaned and weighed after the immersion test did not show any film formation. On the other hand, the polarization resistance showed a constant value or an increase after a decrease immediately after immersion. It is suggested that the change in corrosion rate is affected by the formation of film by immersion, since the value of polarization resistance is almost the same as the sum of film resistance and charge transfer resistance. The film was considered to be mainly Na Ta
Ta O
O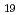 formed by the dissolution of Ta.
formed by the dissolution of Ta.
 in NaCl
in NaClAoyama, Takahito; Ogawa, Hiroaki; Kato, Chiaki; Ueno, Fumiyoshi
Metals, 11(3), p.511_1 - 511_13, 2021/03
Times Cited Count:3 Percentile:16.41(Materials Science, Multidisciplinary)The effect of Cu in bulk solution on pitting corrosion resistance of extra high purity type 316 stainless steel was investigated. Pitting occurred in 0.1 M NaCl-1 mM CuCl
in bulk solution on pitting corrosion resistance of extra high purity type 316 stainless steel was investigated. Pitting occurred in 0.1 M NaCl-1 mM CuCl whereas pitting was not initiated in 0.1 M NaCl. Although deposition of Cu
whereas pitting was not initiated in 0.1 M NaCl. Although deposition of Cu on the surface occurred regardless of potential region in 0.1 M NaCl-1 mM CuCl
on the surface occurred regardless of potential region in 0.1 M NaCl-1 mM CuCl , Cu
, Cu in bulk solution had no influence on the passive film formation. The decrease in pitting corrosion resistance in 0.1 M NaCl-1 mM CuCl
in bulk solution had no influence on the passive film formation. The decrease in pitting corrosion resistance in 0.1 M NaCl-1 mM CuCl resulted from the deposited Cu or Cu compound and continuous supply of Cu
resulted from the deposited Cu or Cu compound and continuous supply of Cu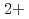 on the surface.
on the surface.
Hashikura, Yasuaki*; Ishijima, Yasuhiro; Nakahara, Masaumi; Sano, Yuichi; Ueno, Fumiyoshi; Abe, Hitoshi
Hozengaku, 19(3), p.95 - 102, 2020/10
A plutonium concentrator was selected, and constant load tensile tests with controlled applied potentials and electrochemical tests were conducted in nitric acid and sodium nitrate solutions. From the results, a map which shows the effect of nitric acid concentration to crack initiation potential was drawn. And, it was pointed out that not only the nitric acid but also the nitrate ion coordinated to the nitrate must be considered in evaluating the possibility of stress corrosion cracking.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 69(9), p.246 - 252, 2020/09
The purpose of this study was to investigate the effect of artificial sea water concentration on the corrosion rate of carbon steel under air/solution alternating condition, and to clarify the corrosion mechanism of carbon steel that changes with artificial seawater concentration. Mass measurements showed that the corrosion rate of carbon steel in the alternating condition accelerates with increasing concentration in the concentration region between deionized water to 200 times diluted artificial seawater (ASW), and the corrosion rate decreases with increasing concentration in the concentration region between 20 times diluted ASW to undiluted ASW. It can be considered that the reason why the carbon steel corrosion was suppressed in highly concentrated artificial seawater would Mg ions and Ca ions in the artificial seawater precipitate and cover on the surface due to the increase in pH near the surface by oxygen reduction reaction.