Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Ueno, Katsuhiro; Watanabe, Masayuki
Scientific Reports (Internet), 15, p.18515_1 - 18515_7, 2025/05
Times Cited Count:0We first demonstrate a nonaqueous rechargeable battery using uranium and iron as active materials. This uranium-iron battery achieves an open-circuit voltage of approximately 1.3 V, exhibits stable cycling performance, and delivers a good Coulombic efficiency of 86 2%. These characteristics suggest a promising avenue for utilizing depleted uranium in innovative applications.
2%. These characteristics suggest a promising avenue for utilizing depleted uranium in innovative applications.
 and SrLaCuNbO
and SrLaCuNbO ; Spin-
; Spin-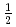 quasi-square-lattice
quasi-square-lattice 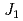 -
-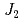 Heisenberg antiferromagnets
Heisenberg antiferromagnetsWatanabe, Masari*; Kurita, Nubuyuki*; Tanaka, Hidekazu*; Ueno, Wataru*; Matsui, Kazuki*; Goto, Takayuki*; Hagihara, Masato
Physical Review B, 105(5), p.054414_1 - 054414_12, 2022/02
Times Cited Count:7 Percentile:53.46(Materials Science, Multidisciplinary)Ueno, Kazuki*; Suzuki, Hiroshi; Takamura, Masato*; Nishio, Yuhei*; Kanematsu, Manabu*
Konkurito Kozobutsu No Hoshu, Hokyo, Appuguredo Rombun Hokokushu (CD-ROM), 20, p.273 - 278, 2020/10
no abstracts in English
Ueno, Kazuki*; Suzuki, Hiroshi; Koyama, Taku*; Nishio, Yuhei*; Kanematsu, Manabu*
Konkurito Kozobutsu No Hoshu, Hokyo, Appuguredo Rombun Hokokushu (CD-ROM), 18, p.647 - 650, 2018/10
no abstracts in English
Koyama, Taku*; Ueno, Kazuki*; Sekine, Mariko*; Matsumoto, Yoshihiro*; Kai, Tetsuya; Shinohara, Takenao; Iikura, Hiroshi; Suzuki, Hiroshi; Kanematsu, Manabu*
Materials Research Proceedings, Vol.4, p.155 - 160, 2018/05
Times Cited Count:0 Percentile:0.00(Materials Science, Characterization & Testing)Yokota, Yu*; Ouchi, Kazuki; Ueno, Katsuhiro; Takao, Koichiro*; Watanabe, Masayuki
no journal, ,
The electrode reaction of ferrocene/ferrocenium ion in ionic liquid-organic solvent mixtures was investigated for the application of non-aqueous solvents to redox flow batteries. The reversibility of the electrode reaction and the diffusion coefficient of ferrocene were measured to evaluate the mixing ratio of ionic liquids and organic solvents. As a result, the ionic liquid concentration suitable for the redox flow battery was found to be about 0.5 M (Volume ratio: 13%).
Yokota, Yu*; Ouchi, Kazuki; Ueno, Katsuhiro; Takao, Koichiro*; Watanabe, Masayuki
no journal, ,
no abstracts in English
Ueno, Kazuki*; Suzuki, Hiroshi; Koyama, Taku*; Sekine, Mariko*; Nishio, Yuhei*; Kanematsu, Manabu*
no journal, ,
no abstracts in English
Sekine, Mariko*; Suzuki, Hiroshi; Koyama, Taku*; Ueno, Kazuki*; Nishio, Yuhei*; Kanematsu, Manabu*
no journal, ,
no abstracts in English
Ueno, Katsuhiro; Ouchi, Kazuki; Watanabe, Masayuki
no journal, ,
For using uranium as active material in redox-flow batteries, we investigated the dependence of chloride ion concentration on the redox reaction of uranium chloride (IV) in an apricot organic solvent. The standard rate constant of the redox reaction increased about 5 times by increasing the chloride ion concentration from 0.1 mol/L to 1 mol/L, improving the redox reaction's reversibility.
Ouchi, Kazuki; Komatsu, Atsushi; Ueno, Katsuhiro; Kitatsuji, Yoshihiro; Watanabe, Masayuki
no journal, ,
The redox reaction of uranium (IV) iodide in an ionic liquid-DMF mixture was investigated as part of a study of uranium reactions in non-aqueous solutions. We found that U(III)/U(IV) reaction is enhanced in reversibility by the addition of iodide ions.