Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Hideya; Tsubata, Yasuhiro; Kurosawa, Tatsuya; Shibata, Mitsunobu; Kawasaki, Tomohiro; Urabe, Shunichi*; Matsumura, Tatsuro
Analytical Sciences, 32(4), p.477 - 479, 2016/04
Times Cited Count:29 Percentile:71.28(Chemistry, Analytical)An impeccable, high-performance new reagent called alkyl diamide amine (ADAAM) was examined from the viewpoint of mutual separation of Am(III) and Eu(III). ADAAM has three donor atoms, one soft N-donor atom and two hard O-donor atoms, in the central frame. The combination of soft and hard atoms affords a tridentate donor set of atoms that ensures remarkable extractability and selectivity of Am(III) and Eu(III) in highly acidic media.
 ,
, -dialkylamides-nitric acid systems
-dialkylamides-nitric acid systemsTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Urabe, Shunichi; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1153 - 1157, 2015/09
 ,
, -Dialkylamides are promising alternative extractants to tri-
-Dialkylamides are promising alternative extractants to tri- -butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.
-butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.  ,
, -Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in
-Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in  -dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
-dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
 nitric acid at elevated temperatures
nitric acid at elevated temperaturesBan, Yasutoshi; Hakamatsuka, Yasuyuki; Tsutsui, Nao; Urabe, Shunichi; Hagiya, Hiromichi; Matsumura, Tatsuro
Radiochimica Acta, 102(9), p.775 - 780, 2014/09
Times Cited Count:5 Percentile:34.55(Chemistry, Inorganic & Nuclear)Optical absorption spectra of Np in 3 mol/dm nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI),
nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI), 
 , were obtained at various temperature. The values of
, were obtained at various temperature. The values of 
 was found to decrease with increasing the temperature, and could be described by the equation
was found to decrease with increasing the temperature, and could be described by the equation 
 = -0.14
= -0.14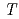 +85.5, where
+85.5, where 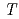 is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm
is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)]
nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)] /dt = 2.2
/dt = 2.2 10
10 exp[-65
exp[-65 10
10 /(
/(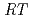 )][Np(V)]
)][Np(V)] , where
, where  and [Np(V)]
and [Np(V)] indicate the gas constant and Np(V) concentration at time
indicate the gas constant and Np(V) concentration at time 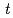 , respectively.
, respectively.
鈴木 英哉; 松村 達郎; ト部 峻一; 黒澤 達也; 川崎 倫弘
国井 茂*; 吉田 貴昌*; 成田 弘一*; 田中 幹也*
【課題】原子力分野や鉱工業分野で利用できるアクチノイドやランタノイドの効率的な抽出方法を提供することを目的とする。 【解決手段】アクチノイド及び/又はランタノイドを含む酸性水溶液を、下記一般式(A)(化学式のため省略)で表されるイミノ二酢酸ジアミドの存在下で有機溶媒に接触させることにより、アクチノイド及び/又はランタノイドを有機溶媒に溶解させて、効率良く抽出することができる。
 ,
, -dialkylamides-nitric acid systems by turbidity measurement, 2; The Correlation between turbidity and the distribution ratio of uranium
-dialkylamides-nitric acid systems by turbidity measurement, 2; The Correlation between turbidity and the distribution ratio of uraniumTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Urabe, Shunichi; Matsumura, Tatsuro
no journal, ,
The turbidity of the agitated solutions of  ,
, -di(2-ethylhexyl)-butanamide (DEHBA) in dodecane and uranyl nitrate in 3 M HNO
-di(2-ethylhexyl)-butanamide (DEHBA) in dodecane and uranyl nitrate in 3 M HNO was measured for 30 min. The variation of the distribution ratio of uranium after stopping the agitation was also measured, and the correlation between the turbidity and the distribution ratio was studied. The turbidity and the distribution ratio of uranium at 3 min after stopping the agitation was more than 1000 FTU and 1.7, respectively, when the initial uranium concentration was 700 mM. The turbidity at 6 min decreased to 363 FTU, while the distribution rate increased to 2.2. Although the turbidity after 6 min decreased gradually, the distribution ratio barely changed. The correlation between the turbidity and the distribution ratio is discussed.
was measured for 30 min. The variation of the distribution ratio of uranium after stopping the agitation was also measured, and the correlation between the turbidity and the distribution ratio was studied. The turbidity and the distribution ratio of uranium at 3 min after stopping the agitation was more than 1000 FTU and 1.7, respectively, when the initial uranium concentration was 700 mM. The turbidity at 6 min decreased to 363 FTU, while the distribution rate increased to 2.2. Although the turbidity after 6 min decreased gradually, the distribution ratio barely changed. The correlation between the turbidity and the distribution ratio is discussed.
Matsumura, Tatsuro; Tsubata, Yasuhiro; Urabe, Shunichi; Ichimura, Seiji; Hagiya, Hiromichi; Tsujimoto, Kazufumi
no journal, ,
no abstracts in English
Urabe, Shunichi; Tsubata, Yasuhiro; Suzuki, Hideya; Shibata, Mitsunobu; Kurosawa, Tatsuya; Kawasaki, Tomohiro; Matsumura, Tatsuro
no journal, ,
JAEA continues to carry out the development of the ADS (Double-strata). Mutual separation of MA and Ln is important for the development of the partitioning process. TDdDGA was performed using a mixer-settler equipment for the development of the separation process for MA. TDdDGA has a high extraction capacity. TDdDGA consists of C, H, O and N atoms (the CHON-principle). In this experiment, the feed solution as simulated HLLW contained fourteen elements. The results of the counter-current test showed that Am was extracted and was recovered in a high yield.
Suzuki, Hideya; Tsubata, Yasuhiro; Urabe, Shunichi; Shibata, Mitsunobu; Kurosawa, Tatsuya; Kawasaki, Tomohiro; Matsumura, Tatsuro
no journal, ,
JAEA continues to carry out the development of the ADS(Double-strata). Mutual separation of MA and Ln is important for the development of the partitioning process. The separation is particularly difficult, because they have the analogous chemical behavior. New reagent was synthesized and examined. The results of separation factors (SF) of Am against Eu are more than 25. The reagent is very stable chemically, high extraction capacity, fully miscible with hydrocarbon diluents, fast chemical kinetics, and following the CHON-principle.
Matsumura, Tatsuro; Tsubata, Yasuhiro; Urabe, Shunichi; Shibata, Mitsunobu; Ichimura, Seiji; Hagiya, Hiromichi*; Tsujimoto, Kazufumi
no journal, ,
no abstracts in English