Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Vauchy, R.; Hirooka, Shun; Saito, Kosuke
Materials Today Communications (Internet), 41, p.110676_1 - 110676_17, 2024/12
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Oxygen potential measurements of PuO reported in the open literature were reviewed and re-interpreted using the defect chemistry model developed by our team. An empirical, easy-to-use, relationship connecting the O/Pu ratio, the equilibrium oxygen potential, and the temperature is proposed based on the interpolation of the experimental data in the 953-2100 K temperature range. The effect of americium on the oxygen potential of PuO
reported in the open literature were reviewed and re-interpreted using the defect chemistry model developed by our team. An empirical, easy-to-use, relationship connecting the O/Pu ratio, the equilibrium oxygen potential, and the temperature is proposed based on the interpolation of the experimental data in the 953-2100 K temperature range. The effect of americium on the oxygen potential of PuO is also discussed.
is also discussed.
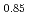 Am
Am O
O at 1473, 1573, and 1673 K
at 1473, 1573, and 1673 KWatanabe, Masashi; Yokoyama, Keisuke; Vauchy, R.; Kato, Masato; Sugata, Hiromasa*; Seki, Takayuki*; Hino, Tetsushi*
Journal of Nuclear Materials, 599, p.155232_1 - 155232_5, 2024/10
Times Cited Count:2 Percentile:75.80(Materials Science, Multidisciplinary)Oxygen potential data of U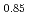 Am
Am O
O were measured at 1473, 1573, and 1673 K by thermogravimetry. In U
were measured at 1473, 1573, and 1673 K by thermogravimetry. In U An
An O
O , where An stands for Pu or Am, and for a given value of y and Oxygen/Metal ratio, the oxygen potential of U
, where An stands for Pu or Am, and for a given value of y and Oxygen/Metal ratio, the oxygen potential of U Am
Am O
O is higher than that of U
is higher than that of U Pu
Pu O
O . The valence of cations in the hypostoichiometric region is similar to that of Nd-doped UO
. The valence of cations in the hypostoichiometric region is similar to that of Nd-doped UO . At the stoichiometric composition, it is estimated to be Am
. At the stoichiometric composition, it is estimated to be Am , U
, U , and U
, and U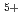 (for charge compensation of Am
(for charge compensation of Am ). The experimental data were analyzed using a defect chemistry model, and a relationship connecting the oxygen-to-metal ratio, the temperature, and the equilibrium oxygen partial pressure was proposed.
). The experimental data were analyzed using a defect chemistry model, and a relationship connecting the oxygen-to-metal ratio, the temperature, and the equilibrium oxygen partial pressure was proposed.
Vauchy, R.; Hirooka, Shun; Horii, Yuta; Ogasawara, Masahiro*; Sunaoshi, Takeo*; Yamada, Tadahisa*; Tamura, Tetsuya*; Murakami, Tatsutoshi
Journal of Nuclear Materials, 599, p.155233_1 - 155233_11, 2024/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)The fluorite exsolution/recombination in U Pu
Pu O
O (y = 0.30 and 0.45) and PuO
(y = 0.30 and 0.45) and PuO was investigated using differential scanning calorimetry. The results are in relatively good agreement with the literature data, except for plutonia. Our values indicate that the critical temperature of the miscibility gap in Pu-O is 30
was investigated using differential scanning calorimetry. The results are in relatively good agreement with the literature data, except for plutonia. Our values indicate that the critical temperature of the miscibility gap in Pu-O is 30 50 K lower than previously reported. Finally, the systematic experimental procedure allowed us refining the locus of the solvus existing in hypostoichiometric U
50 K lower than previously reported. Finally, the systematic experimental procedure allowed us refining the locus of the solvus existing in hypostoichiometric U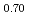 0Pu
0Pu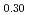 O
O , U
, U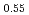 Pu
Pu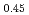 O
O , and PuO
, and PuO dioxides.
dioxides.
 Pu
Pu O
O powder using master sintering curve theory
powder using master sintering curve theoryNakamichi, Shinya; Sunaoshi, Takeo*; Hirooka, Shun; Vauchy, R.; Murakami, Tatsutoshi
Journal of Nuclear Materials, 595, p.155072_1 - 155072_11, 2024/07
Times Cited Count:1 Percentile:51.66(Materials Science, Multidisciplinary)Kato, Masato; Oki, Takumi; Watanabe, Masashi; Hirooka, Shun; Vauchy, R.; Ozawa, Takayuki; Uwaba, Tomoyuki; Ikusawa, Yoshihisa; Nakamura, Hiroki; Machida, Masahiko
Journal of the American Ceramic Society, 107(5), p.2998 - 3011, 2024/05
Times Cited Count:5 Percentile:41.38(Materials Science, Ceramics) mixed oxides
mixed oxidesVauchy, R.; Matsumoto, Taku; Hirooka, Shun; Uno, Hiroki*; Tamura, Tetsuya*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Nakamura, Hiroki; Machida, Masahiko; et al.
Journal of Nuclear Materials, 588, p.154786_1 - 154786_13, 2024/01
Times Cited Count:6 Percentile:84.29(Materials Science, Multidisciplinary)Vauchy, R.
Kaku Nenryo, (59-1), p.13 - 15, 2023/12
Vauchy, R.; Hirooka, Shun; Murakami, Tatsutoshi
Materialia, 32, p.101934_1 - 101934_12, 2023/12
Vauchy, R.; Hirooka, Shun; Murakami, Tatsutoshi
Materialia, 32, p.101943_1 - 101943_8, 2023/12
Hirooka, Shun; Horii, Yuta; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*; Vauchy, R.; Hayashizaki, Kohei; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Science and Technology, 60(11), p.1313 - 1323, 2023/11
Times Cited Count:5 Percentile:78.63(Nuclear Science & Technology)Additive MOX pellets are fabricated by a conventional dry powder metallurgy method. Nd O
O and Sm
and Sm O
O are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
Vauchy, R.; Hirooka, Shun; Watanabe, Masashi; Yokoyama, Keisuke; Murakami, Tatsutoshi
Journal of Nuclear Materials, 584, p.154576_1 - 154576_11, 2023/10
Times Cited Count:6 Percentile:84.29(Materials Science, Multidisciplinary)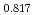 Pu
Pu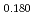 Am
Am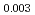 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:9 Percentile:93.25(Materials Science, Multidisciplinary)Vauchy, R.; Hirooka, Shun; Watanabe, Masashi; Kato, Masato
Scientific Reports (Internet), 13, p.2217_1 - 2217_8, 2023/02
Times Cited Count:10 Percentile:72.61(Multidisciplinary Sciences) ceramics
ceramicsVauchy, R.; Hirooka, Shun; Watanabe, Masashi; Yokoyama, Keisuke; Sunaoshi, Takeo*; Yamada, Tadahisa*; Nakamichi, Shinya; Murakami, Tatsutoshi
Ceramics International, 49(2), p.3058 - 3065, 2023/01
Times Cited Count:11 Percentile:63.47(Materials Science, Ceramics) , ThO
, ThO , UO
, UO , PuO
, PuO , and (U, Pu)O
, and (U, Pu)O
Kato, Masato; Watanabe, Masashi; Hirooka, Shun; Vauchy, R.
Frontiers in Nuclear Engineering (Internet), 1, p.1081473_1 - 1081473_10, 2023/01
Vauchy, R.; Hirooka, Shun; Matsumoto, Taku; Kato, Masato
Frontiers in Nuclear Engineering (Internet), 1, p.1060218_1 - 1060218_18, 2022/12
Hirooka, Shun; Vauchy, R.; Horii, Yuta; Akashi, Masatoshi; Sunaoshi, Takeo*; Saito, Kosuke
no journal, ,
no abstracts in English
Vauchy, R.
no journal, ,
A comparison of the French and Japanese nuclear fuel cycles is proposed with a focus on MOX fuel production for LWRs and SFRs.
Vauchy, R.; Hirooka, Shun; Sunaoshi, Takeo*; Kato, Masato
no journal, ,
Kato, Masato; Hirooka, Shun; Watanabe, Masashi; Vauchy, R.; Oki, Takumi
no journal, ,
no abstracts in English