Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hanamachi, Yuji*; Walker, C.*; Sasamoto, Hiroshi; Mihara, Morihiro
NIMS Bisai Kozo Kaiseki Purattofuomu Riyo Hokokusho (Internet), 2 Pages, 2023/12
A High-volume Fly ash Silica fume Cement (HFSC) has been considered as the use of concrete support of drift in deep disposal of radioactive wastes. JAEA has been carried out experiments and modeling studies to evaluate chemical stability of C-A-S-H gel synthesized which would be believed as the dominant component of the HFSC. For the modeling purpose, it is necessary to evaluate the chemical composition both of C-A-S-H gel synthesized and accompanied minerals in the experiment. In the year of 2020, 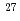 Al and
Al and 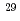 Si NMR measurement were performed to identify Al and Si distribution in the sample and chemical composition of C-A-S-H gel was derived. However, it was impossible to derive the C-A-S-H gel composition having the expected Al/Si molar ratio since there were several accompanied minerals produced in the experiment due to the short term of immersion. This time similar approach to derive C-A-S-H gel composition is adopted using NMR measurement for the sample of longer time (6 months).
Si NMR measurement were performed to identify Al and Si distribution in the sample and chemical composition of C-A-S-H gel was derived. However, it was impossible to derive the C-A-S-H gel composition having the expected Al/Si molar ratio since there were several accompanied minerals produced in the experiment due to the short term of immersion. This time similar approach to derive C-A-S-H gel composition is adopted using NMR measurement for the sample of longer time (6 months).
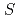 =
= 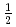 quasikagome-lattice compound CeRh
quasikagome-lattice compound CeRh Pd
Pd Sn investigated using muon spin relaxation and neutron scattering
Sn investigated using muon spin relaxation and neutron scatteringTripathi, R.*; Adroja, D. T.*; Ritter, C.*; Sharma, S.*; Yang, C.*; Hillier, A. D.*; Koza, M. M.*; Demmel, F.*; Sundaresan, A.*; Langridge, S.*; et al.
Physical Review B, 106(6), p.064436_1 - 064436_17, 2022/08
Times Cited Count:2 Percentile:34.67(Materials Science, Multidisciplinary)Walker, C.*
NIMS Bisai Kozo Kaiseki Purattofuomu Riyo Hokokusho (Internet), 2 Pages, 2021/09
High content fly ash silica fume cement (HFSC) has been considering a candidate low alkali cements for the geological disposal of radioactive waste in Japan. JAEA has been currently performing many experiments and modeling studies relevant to validation for the long-term stability of the C-A-S-H gel which is a dominant component of the HFSC. For developing the C-A-S-H model of hydration and degradation, it is necessary to determine the composition of C-A-S-H gel and accompanied minerals when it reacts with water. In the present subject, the synthesized sample of C-A-S-H gels were analyzed by using the NMR spectra of 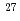 Al and
Al and 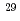 Si to determine the coordination of these element in the C-A-S-H gel.
Si to determine the coordination of these element in the C-A-S-H gel.
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 195, p.105741_1 - 105741_11, 2020/09
Times Cited Count:2 Percentile:10.08(Chemistry, Physical)Safety functions for the clay buffer in a repository for high-level radioactive waste (HLW) are fulfilled if the presence of montmorillonite with high swelling capacity and low permeability is maintained in the long-term. The transformation of montmorillonite to the non-swelling mineral likely illite is addressed in most safety assessments by using simple semi-empirical kinetic models, but this approach contrasts with more complex reactive-transport simulations. In the present study, reactive-transport simulations are compared with simple semi-empirical kinetic models. Results suggest that reactive-transport simulations err on the side of conservatism, but may produce unrealistic estimates of illitization. This comparison demonstrates that reactive-transport models may be carefully applied to simulate the long-term evolution of near field environment for HLW disposal.
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 179, p.105146_1 - 105146_10, 2019/10
Times Cited Count:13 Percentile:62.41(Chemistry, Physical)Natural systems evidence for the effects of temperature and the activity of aqueous silica upon montmorillonite stability was evaluated. Thermodynamic modeling using three different TDBs shows that stability fields for montmorillonite exist from 0 to 140 C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60
C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60 C to that for illite + quartz at higher temperatures. However, even over very long timescales (
C to that for illite + quartz at higher temperatures. However, even over very long timescales ( 1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80
1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80 C.
C.
Anraku, Sohtaro; Walker, C.*; Oda, Chie; Mihara, Morihiro; Honda, Akira
Proceedings of 15th International Congress on the Chemistry of Cement (ICCC 2019) (Internet), 11 Pages, 2019/09
Walker, C.*; Anraku, Sohtaro; Oda, Chie; Mihara, Morihiro; Honda, Akira
Proceedings of 15th International Congress on the Chemistry of Cement (ICCC 2019) (Internet), 11 Pages, 2019/09

 = 0 selection rule in Gamow-Teller transitions; The
= 0 selection rule in Gamow-Teller transitions; The  -decay of
-decay of 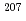 Hg
HgBerry, T. A.*; Podoly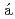 k, Zs.*; Carroll, R. J.*; Lic
k, Zs.*; Carroll, R. J.*; Lic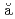 , R.*; Grawe, H.*; Timofeyuk, N. K.*; Alexander, T.*; Andreyev, A. N.; Ansari, S.*; Borge, M. J. G.*; et al.
, R.*; Grawe, H.*; Timofeyuk, N. K.*; Alexander, T.*; Andreyev, A. N.; Ansari, S.*; Borge, M. J. G.*; et al.
Physics Letters B, 793, p.271 - 275, 2019/06
Times Cited Count:5 Percentile:47.88(Astronomy & Astrophysics) C based on two binary non-ideal solid solutions
C based on two binary non-ideal solid solutionsWalker, C.; Suto, Shunkichi; Oda, Chie; Mihara, Morihiro; Honda, Akira
Cement and Concrete Research, 79, p.1 - 30, 2016/01
Times Cited Count:69 Percentile:90.65(Construction & Building Technology)Modeling the solubility behavior of calcium silicate hydrate (C-S-H) gel is important to make quantitative predictions of the degradation of hydrated ordinary Portland cement (OPC) based materials. Experimental C-S-H gel solubility data have been compiled from the literature, critically evaluated and supplemented with new data from the current study for molar Ca/Si ratios = 0.2-0.83. All these data have been used to derive a discrete solid phase (DSP) type C-S-H gel solubility model based on two binary non-ideal solid solutions in aqueous solution(SSAS). Features of the DSP type C-S-H gel solubility model include satisfactory predictions of pH values and Ca and Si concentrations for all molar Ca/Si ratios = 2.7  0 in the C-S-H system, portlandite (CH) for Ca/Si ratios
0 in the C-S-H system, portlandite (CH) for Ca/Si ratios  1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO
1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO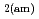 ) for Ca/Si ratios
) for Ca/Si ratios  0.55 as identified in the current study by IR spectroscopy.
0.55 as identified in the current study by IR spectroscopy.
 C
COda, Chie; Walker, C.; Chino, Daisuke*; Ichige, Satoru; Honda, Akira; Sato, Tsutomu*; Yoneda, Tetsuro*
Applied Clay Science, 93-94, p.62 - 71, 2014/05
Times Cited Count:7 Percentile:24.59(Chemistry, Physical)Na-montmorillonite dissolution in a 0.3M NaOH solution has been investigated at pH12 and 70 C. The flow-through dissolution experiments were conducted in a dispersed system with varying concentrations of Si and Al to derive a Na-montmorillonite dissolution rate, as a non-linear function of the Gibbs free energy of reaction, dGr. This rate equation was used to simulate the batch-type Na-montmorillonite reaction experiments conducted in a coagulated system. The model simulation of the batch-type experiment adopting the empirical rate equations of Na-montmorillonite dissolution and secondary mineral analcime precipitation were able to reproduce the measured changes in the amount of dissolved Na-montmorillonite and concentrations of Si and Al in solution. The results showed that the empirical rate equation of Na-montmorillonite dissolution determined in the dispersed system was applicable to the coagulated system over a higher dGr range and that the concentrations of Si and Al in the batch experiment were controlled by the precipitation of analcime.
C. The flow-through dissolution experiments were conducted in a dispersed system with varying concentrations of Si and Al to derive a Na-montmorillonite dissolution rate, as a non-linear function of the Gibbs free energy of reaction, dGr. This rate equation was used to simulate the batch-type Na-montmorillonite reaction experiments conducted in a coagulated system. The model simulation of the batch-type experiment adopting the empirical rate equations of Na-montmorillonite dissolution and secondary mineral analcime precipitation were able to reproduce the measured changes in the amount of dissolved Na-montmorillonite and concentrations of Si and Al in solution. The results showed that the empirical rate equation of Na-montmorillonite dissolution determined in the dispersed system was applicable to the coagulated system over a higher dGr range and that the concentrations of Si and Al in the batch experiment were controlled by the precipitation of analcime.
Pitcher, C. S.*; Barnsley, R.*; Bertalot, L.*; Encheva, A.*; Feder, R.*; Friconneau, J. P.*; Hu, Q.*; Levesy, B.*; Loesser, G. D.*; Lyublin, B.*; et al.
Fusion Science and Technology, 64(2), p.118 - 125, 2013/08
Times Cited Count:4 Percentile:32.48(Nuclear Science & Technology)The port-based plasma diagnostic infrastructure on ITER is described, including the port plugs, the interspace support structure and port cell structure. These systems are modular in nature with standardized dimensions. The design of the equatorial and upper port plugs and their modules is discussed, as well as the dominant loading mechanisms. The port infrastructure design has now matured to the point that port plugs are now being populated with multiple diagnostics supplied by a number of ITER partners - two port plug examples are given.
Tsuda, Hidenori; Walker, C.; Shinkai, Fumiaki*; Kishi, Hirokazu*; Yui, Mikazu
Journal of Nuclear Science and Technology, 49(11), p.1110 - 1113, 2012/11
Times Cited Count:4 Percentile:31.96(Nuclear Science & Technology)Fujita, Tomoo; Kawaguchi, Masanao; Walker, C.; Sasamoto, Hiroshi; Yui, Mikazu; Onishi, Yuzo*
Proceedings of 7th Asian Rock Mechanics Symposium (ARMS-7) (USB Flash Drive), p.675 - 681, 2012/10
The Japan Atomic Energy Agency started new grout project for geological disposal of high-level radioactive waste in 2007. This study presented the overall JAEA grout project program and an example of how to apply key engineering technologies to the construction and operation of an underground facility for the geological disposal of HLW.
Pitcher, C. S.*; Barnsley, R.*; Feder, R.*; Hu, Q.*; Loesser, G. D.*; Lyublin, B.*; Padasalagi, S.*; Pak, S.*; Reichle, R.*; Sato, Kazuyoshi; et al.
Fusion Engineering and Design, 87(5-6), p.667 - 674, 2012/08
Times Cited Count:12 Percentile:66.54(Nuclear Science & Technology)Walker, C.; Yui, Mikazu
NEA/RWM/R(2012)3/REV, p.159 - 163, 2012/03
no abstracts in English
Arthur, R. C.*; Sasamoto, Hiroshi; Walker, C.; Yui, Mikazu
Clays and Clay Minerals, 59(6), p.626 - 639, 2011/11
Times Cited Count:16 Percentile:45.29(Chemistry, Physical)Regarding to geological disposal of high level radioactive waste, long-term evolution of chemical condition in the rock mass by interactions of cementitious grout and rock would be predicted, if the grout is used for reducing the groundwater inflow during construction of drifts. Evolution of chemical condition is important because it could affect the evaluation of radionuclides migration for performance assessment. Zeolite is one of important alteration minerals by interactions of cementitious grout and rock. For evaluation of long-term evolution of chemical condition, it is necessary to develop thermodynamic data for alteration minerals like zeolite. The present paper proposes a revised model to derive more reliable thermodynamic data for zeolite. Additionally, a possibility to apply this revised model on other important alteration minerals is suggested.
Sato, Kazuyoshi; Yaguchi, Eiji; Pitcher, C. S.*; Walker, C.*; Encheva, A.*; Kawano, Yasunori; Kusama, Yoshinori
Fusion Engineering and Design, 86(6-8), p.1264 - 1267, 2011/10
Times Cited Count:3 Percentile:26.02(Nuclear Science & Technology)no abstracts in English
Vayakis, G.*; Bertalot, L.*; Encheva, A.*; Walker, C.*; Brichard, B.*; Cheon, M. S.*; Chitarin, G.*; Hodgson, E.*; Ingesson, C.*; Ishikawa, Masao; et al.
Journal of Nuclear Materials, 417(1-3), p.780 - 786, 2011/10
Times Cited Count:27 Percentile:87.94(Materials Science, Multidisciplinary) Pb beam
Pb beamSteer, S. J.*; Podoly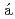 k, Z.*; Pietri, S.*; G
k, Z.*; Pietri, S.*; G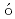 rska, M.*; Grawe, H.*; Maier, K.*; Regan, P. H.*; Rudolph, D.*; Garnsworthy, A. B.*; Hoischen, R.*; et al.
rska, M.*; Grawe, H.*; Maier, K.*; Regan, P. H.*; Rudolph, D.*; Garnsworthy, A. B.*; Hoischen, R.*; et al.
Physical Review C, 84(4), p.044313_1 - 044313_22, 2011/10
Times Cited Count:65 Percentile:94.74(Physics, Nuclear)Heavy neutron-rich nuclei were populated via the fragmentation of a E/A=1 GeV 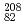 Pb beam. Secondary fragments were separated and identified and subsequently implanted in a passive stopper. By the detection of delayed
Pb beam. Secondary fragments were separated and identified and subsequently implanted in a passive stopper. By the detection of delayed  rays, isomeric decays associated with these nuclei have been identified. A total of 49 isomers were detected, with the majority of them observed for the first time. Possible level schemes are constructed and the structure of the nuclei discussed. To aid the interpretation, shell-model as well as BCS calculations were performed.
rays, isomeric decays associated with these nuclei have been identified. A total of 49 isomers were detected, with the majority of them observed for the first time. Possible level schemes are constructed and the structure of the nuclei discussed. To aid the interpretation, shell-model as well as BCS calculations were performed.
Pitcher, C. S.*; Andrew, P.*; Barnsley, R.*; Bertalot, L.*; Counsell, G. G.*; Encheva, A.*; Feder, R. E.*; Hatae, Takaki; Johnson, D. W.*; Kim, J.*; et al.
Journal of Nuclear Materials, 415(Suppl.1), p.S1127 - S1132, 2011/08
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)