Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 /4H-SiC(11
/4H-SiC(11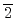 0) interfaces
0) interfacesNakanuma, Takato*; Iwakata, Yu*; Watanabe, Arisa*; Hosoi, Takuji*; Kobayashi, Takuma*; Sometani, Mitsuru*; Okamoto, Mitsuo*; Yoshigoe, Akitaka; Shimura, Takayoshi*; Watanabe, Heiji*
Japanese Journal of Applied Physics, 61(SC), p.SC1065_1 - SC1065_8, 2022/05
Times Cited Count:7 Percentile:77.62(Physics, Applied)Nitridation of SiO /4H-SiC(11
/4H-SiC(11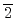 0) interfaces with post-oxidation annealing in an NO ambient (NO-POA) and its impact on the electrical properties were investigated. Sub-nm-resolution nitrogen depth profiling at the interfaces was conducted by using a scanning X-ray photoelectron spectroscopy microprobe. The results showed that nitrogen atoms were incorporated just at the interface and that interface nitridation proceeded much faster than at SiO
0) interfaces with post-oxidation annealing in an NO ambient (NO-POA) and its impact on the electrical properties were investigated. Sub-nm-resolution nitrogen depth profiling at the interfaces was conducted by using a scanning X-ray photoelectron spectroscopy microprobe. The results showed that nitrogen atoms were incorporated just at the interface and that interface nitridation proceeded much faster than at SiO /SiC(0001) interfaces, resulting in a 2.3 times higher nitrogen concentration. Electrical characterizations of metal-oxide-semiconductor capacitors were conducted through capacitance-voltage (
/SiC(0001) interfaces, resulting in a 2.3 times higher nitrogen concentration. Electrical characterizations of metal-oxide-semiconductor capacitors were conducted through capacitance-voltage (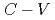 ) measurements in the dark and under illumination with ultraviolet light to evaluate the electrical defects near the conduction and valence band edges and those causing hysteresis and shifting of the
) measurements in the dark and under illumination with ultraviolet light to evaluate the electrical defects near the conduction and valence band edges and those causing hysteresis and shifting of the 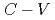 curves. While all of these defects were passivated with the progress of the interface nitridation, excessive nitridation resulted in degradation of the MOS capacitors. The optimal conditions for NO-POA are discussed on the basis of these experimental findings.
curves. While all of these defects were passivated with the progress of the interface nitridation, excessive nitridation resulted in degradation of the MOS capacitors. The optimal conditions for NO-POA are discussed on the basis of these experimental findings.
 -rays
-raysArisaka, Makoto; Watanabe, Masayuki; Ishizaki, Manabu*; Kurihara, Masato*; Chen, R.*; Tanaka, Hisashi*
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1543 - 1547, 2015/02
Times Cited Count:13 Percentile:70.51(Chemistry, Analytical)The influence of irradiation with  -rays to metal hexacyanoferrate (MHCF: M = Fe, Cu or Ni), which is known as an adsorbent for selective adsorption of cesium (Cs) ion in solution, on Cs adsorption ability and stability was investigated in HNO
-rays to metal hexacyanoferrate (MHCF: M = Fe, Cu or Ni), which is known as an adsorbent for selective adsorption of cesium (Cs) ion in solution, on Cs adsorption ability and stability was investigated in HNO solutions. Under the adsorbed dose conditions (50-300 kGy), it was found that the MHCF is fully stable although the radiolytic decomposition of MHCF was slightly observed with an increase of the total adsorbed dose, which was confirmed by an increment of Fe, Cu or Ni concentration in HNO
solutions. Under the adsorbed dose conditions (50-300 kGy), it was found that the MHCF is fully stable although the radiolytic decomposition of MHCF was slightly observed with an increase of the total adsorbed dose, which was confirmed by an increment of Fe, Cu or Ni concentration in HNO solution after the irradiation. The weight percent of the metal in the solution to initial weight of MHCF was less than unity. Moreover, no change in composition of carbon, hydrogen and nitrogen in MHCF was observed. On the other hand, the distribution coefficients of Cs to the irradiated MHCF were independent of the total adsorbed dose. This indicates that the Cs adsorption ability was maintained under
solution after the irradiation. The weight percent of the metal in the solution to initial weight of MHCF was less than unity. Moreover, no change in composition of carbon, hydrogen and nitrogen in MHCF was observed. On the other hand, the distribution coefficients of Cs to the irradiated MHCF were independent of the total adsorbed dose. This indicates that the Cs adsorption ability was maintained under  -ray irradiation.
-ray irradiation.
 -ray irradiation
-ray irradiationArisaka, Makoto; Watanabe, Masayuki; Sugo, Yumi; Kobayashi, Kumiko*; Kanao, Osamu*; Kimura, Takaumi
Journal of Nuclear Science and Technology, 51(4), p.457 - 464, 2014/04
Times Cited Count:3 Percentile:23.92(Nuclear Science & Technology)Toward the development of a practical separation method for trivalent actinides and lanthanides, we used extraction chromatography with alkyl-pyridinedicarboxyamides as the extractant. The results confirmed that the performance degradation of the adsorbent caused by contact with HNO and/or irradiation with
and/or irradiation with  rays would be very small during the operation of column chromatography. The optimal conditions for the column separation were also determined: eluent, 5M HNO
rays would be very small during the operation of column chromatography. The optimal conditions for the column separation were also determined: eluent, 5M HNO ; flow rate, 0.1 mL/min.
; flow rate, 0.1 mL/min.
 adsorption
adsorption  lattice defect sites of Prussian blue filled with coordination and crystallization water molecules
lattice defect sites of Prussian blue filled with coordination and crystallization water moleculesIshizaki, Manabu*; Akiba, Sae*; Otani, Asako*; Hoshi, Yuji*; Ono, Kenta*; Matsuba, Mayu*; Togashi, Takanari*; Kanaizuka, Katsuhiko*; Sakamoto, Masatomi*; Takahashi, Akira*; et al.
Dalton Transactions, 42(45), p.16049 - 16055, 2013/12
Times Cited Count:178 Percentile:99.58(Chemistry, Inorganic & Nuclear)We have revealed the fundamental mechanism of specific Cs adsorption into Prussian blue (PB) in order to develop high-performance PB-based Cs
adsorption into Prussian blue (PB) in order to develop high-performance PB-based Cs adsorbents in the wake of the Fukushima nuclear accident. We compared two types of PB nanoparticles with formulae of Fe
adsorbents in the wake of the Fukushima nuclear accident. We compared two types of PB nanoparticles with formulae of Fe
 [Fe
[Fe (CN)
(CN) ]3
]3 xH
xH O (x = 10-15) (PB-1) and (NH
O (x = 10-15) (PB-1) and (NH )0.70Fe
)0.70Fe 1.10[Fe
1.10[Fe (CN)
(CN) ]
] 1.7H
1.7H O (PB-2) with respect to the Cs
O (PB-2) with respect to the Cs adsorption ability. The synthesised PB-1, by a common stoichiometric aqueous reaction between 4Fe
adsorption ability. The synthesised PB-1, by a common stoichiometric aqueous reaction between 4Fe and 3[Fe
and 3[Fe (CN)
(CN) ]
] , showed much more efficient Cs
, showed much more efficient Cs adsorption ability than did the commercially available PB-2.
adsorption ability than did the commercially available PB-2.
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
Radiochimica Acta, 101(11), p.711 - 717, 2013/11
Times Cited Count:3 Percentile:25.73(Chemistry, Inorganic & Nuclear)Four new alkyl-pyridinedicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) were synthesized and used as extractants for partitioning minor actinides from high level radioactive waste using extraction chromatography. The R-PDAs were successfully impregnated into XAD resins (Amberlite). The prepared adsorbents, R-PDA/XAD, exhibited moderate adsorptions of both Am(III) and Eu(III), and separation between Am(III) and Eu(III), over a high HNO concentration range (3-5 M). Deterioration of the prepared adsorbent as a result of contact with HNO
concentration range (3-5 M). Deterioration of the prepared adsorbent as a result of contact with HNO solution was prevented by using R-PDAs containing longer alkyl groups. The equilibrium data for Eu(III) were analyzed using Langmuir and Freundlich isotherm models. The adsorption of Eu(III) ions onto the R-PDA/XAD was fitted better to the Langmuir isotherm than to the Freundlich isotherm. The maximum adsorption capacity was determined to be 24.2 mg Eu/g (R-PDA/XAD).
solution was prevented by using R-PDAs containing longer alkyl groups. The equilibrium data for Eu(III) were analyzed using Langmuir and Freundlich isotherm models. The adsorption of Eu(III) ions onto the R-PDA/XAD was fitted better to the Langmuir isotherm than to the Freundlich isotherm. The maximum adsorption capacity was determined to be 24.2 mg Eu/g (R-PDA/XAD).
Chen, R.*; Tanaka, Hisashi*; Kawamoto, Toru*; Asai, Miyuki*; Fukushima, Chikako*; Na, H.*; Kurihara, Masato*; Watanabe, Masayuki; Arisaka, Makoto; Nankawa, Takuya
Electrochimica Acta, 87, p.119 - 125, 2013/01
Times Cited Count:108 Percentile:94.92(Electrochemistry)A novel electrochemical adsorption system using a nanoparticle film of copper (II) hexacyanoferrate (III) was proposed for selectively removing cesium from wastewater. This system can be used for cesium separation without extra chemical reagents or any filtration treatment. Cesium uptake and elution can be simply controlled by switching the applied potentials between anodes and cathodes. Data from batch kinetic studies well fitted the intraparticle diffusion equation, reflecting a two-step process: a steepest ascent portion followed by a plateau extending to the equilibrium. The effective cesium removal with a high distribution coefficient (

 5
5 10
10 mL/g) can be adopted in a large pH range from 0.3 to 9.2, and in the presence of several diverse coexisting alkaline cations, suggesting it can be taken as a promising technology for actual nuclear wastewater treatment.
mL/g) can be adopted in a large pH range from 0.3 to 9.2, and in the presence of several diverse coexisting alkaline cations, suggesting it can be taken as a promising technology for actual nuclear wastewater treatment.
Chen, R.*; Tanaka, Hisashi*; Kawamoto, Toru*; Asai, Miyuki*; Fukushima, Chikako*; Kurihara, Masato*; Watanabe, Masayuki; Arisaka, Makoto; Nankawa, Takuya
Electrochemistry Communications, 25, p.23 - 25, 2012/11
Times Cited Count:50 Percentile:79.8(Electrochemistry)We first synthesized water-dispersed nanoparticle copper hexacyanoferrate (CuHCF) ink and then coated its nanoparticles on electrodes to electrochemically remove cesium from wastewater. Cesium uptake and elution can be controlled by switching the potentials between anodes and cathodes. Effective cesium removal can be adopted in a large pH range from 0.2 to 8.9, and in the presence of several diverse coexisting alkaline cations, suggesting that it can be taken as a promising technology for actual radioactive wastewater treatment. The prepared CuHCF nanoparticles can be simply and uniformly coated on electrodes by wet process like conventional printing methods, so any sizes or patterns are feasible at low cost, which indicated the potential as a promising sorption electrode of large size in the columns for sequential removal and recycle of Cs from wastewater.
Kozai, Naofumi; Onuki, Toshihiko; Arisaka, Makoto; Watanabe, Masayuki; Sakamoto, Fuminori; Yamasaki, Shinya; Jiang, M.
Journal of Nuclear Science and Technology, 49(5), p.473 - 478, 2012/05
Times Cited Count:64 Percentile:97.82(Nuclear Science & Technology)Chemical states of radioactive Cs in the contaminated soils by Fukushima Daiichi Nuclear Power Plant accident has been characterized by the desorption experiments using appropriate reagents solutions and size fractionation of the contaminated soils. Approximately 70% of radioactive Cs in the residual fraction were associated with the size fractions larger than the elutriated one, even though mica-like minerals were contained in the elutriated one. These results strongly suggest that radioactive Cs was irreversibly associated with soil components other than mica like minerals in the contaminated soil.
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
Journal of Nuclear Materials, 407(2), p.116 - 118, 2010/12
Times Cited Count:6 Percentile:40.28(Materials Science, Multidisciplinary)Adsorption of trivalent actinides (An(III)) and lanthanides (Ln(III)) in acidic aqueous solution using activated carbons was investigated. Activated carbons exhibited significantly selective adsorption to An(III) over Ln(III) in acidic aqueous solution from about pH 1 to pH 4, independently of the starting materials, activation methods and specific surface areas.
Watanabe, Masayuki; Arisaka, Makoto; Kimura, Takaumi
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 5 Pages, 2010/00
N,N'-dimethyl-N,N'-diphenylpyridine-2,6-dicarboxyamide which contains a pyridyl group between two amidic functional groups exhibited moderate separation ability of actinides(III) from lanthanides(III) at high HNO concentration by solvent extraction. To solve the leakage problem on the extraction chromatography, we reported that newly synthesized N,N'-dialkyl-N,N'-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) exhibited reduction on the leakage of extractant from the impregnated resin depending on the length of alkyl group. The column chromatography of actinides(III) from lanthanides (III) was investigated by using the impregnated resin with Oct-PDA.
concentration by solvent extraction. To solve the leakage problem on the extraction chromatography, we reported that newly synthesized N,N'-dialkyl-N,N'-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) exhibited reduction on the leakage of extractant from the impregnated resin depending on the length of alkyl group. The column chromatography of actinides(III) from lanthanides (III) was investigated by using the impregnated resin with Oct-PDA.
Watanabe, Masayuki; Arisaka, Makoto; Kimura, Takaumi
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1062 - 1065, 2009/09
Adsorption of trivalent actinides (An(III)) and lanthanides (Ln(III)) in acidic aqueous solution with carbon nano-materials including activated carbon, graphite and carbon nanotube were investigated. These carbon nano-materials exhibited the significant selectivity to An(III) from Ln(III) in acidic aqueous solution.
 ,
, '-dialkyl-
'-dialkyl- ,
, '-diphenylpyridine-2,6-dicarboxyamides
'-diphenylpyridine-2,6-dicarboxyamidesArisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
Proceedings of International Conference on Advanced Nuclear Fuel Cycles and Systems (Global 2007) (CD-ROM), p.111 - 113, 2007/09
Four  ,
, '-dialkyl-
'-dialkyl- ,
, '-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) were newly synthesized and were applied to extraction chromatography as extractant to attain the separation of actinides(III) from high level radioactive waste containing lanthanides(III). R-PDA was successfully impregnated into XAD-4 resin. It was found that (i) the leakage of R-PDA from XAD-4 resin was suppressed with an increase of the length of the alkyl groups in R-PDA, while the leakage for each adsorbent resin was promoted with an increase of HNO
'-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) were newly synthesized and were applied to extraction chromatography as extractant to attain the separation of actinides(III) from high level radioactive waste containing lanthanides(III). R-PDA was successfully impregnated into XAD-4 resin. It was found that (i) the leakage of R-PDA from XAD-4 resin was suppressed with an increase of the length of the alkyl groups in R-PDA, while the leakage for each adsorbent resin was promoted with an increase of HNO concentration in the aqueous phase and (ii) Oc-PDA or De-PDA/XAD-4 resin exhibits moderate separation ability of actinides(III) from lanthanides(III) at relatively high HNO
concentration in the aqueous phase and (ii) Oc-PDA or De-PDA/XAD-4 resin exhibits moderate separation ability of actinides(III) from lanthanides(III) at relatively high HNO concentration.
concentration.
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
no journal, ,
no abstracts in English
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
no journal, ,
Separation of actinide(III) with long-term alpha-radiotoxicity from high level radioactive waste containing lanthanide(III) is indispensable to attain transmutation of actinide(III). Extraction chromatography is one of the promising separation techniques for recovering small amounts of target components from a solution. In this study, to attain the separation of actinides(III) from lanthanides(III) by extraction chromatography, N,N'-dialkyl-N,N'-diphenylpyridine-2,6-dicarboxyamides (PDA) was synthesized as the extractants. This extractant system is prospective to selectively recognize actinides(III) over lanthanides(III) since this has nitrogen donor (pyridyl group). The distribution coefficient, Kd, of both Am(III) and Eu(III) increased with an increase of HNO concentration. The separation factor (= Kd(Am)/Kd(Eu)) at 3 M HNO
concentration. The separation factor (= Kd(Am)/Kd(Eu)) at 3 M HNO was determined to be 6.9. From the result of column experiment, it was found that a complete separation between Am(III) and Eu(III) was archived.
was determined to be 6.9. From the result of column experiment, it was found that a complete separation between Am(III) and Eu(III) was archived.
Sasaki, Yuji; Suzuki, Shinichi; Kitatsuji, Yoshihiro; Watanabe, Masayuki; Arisaka, Makoto; Kimura, Takaumi; Ban, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
no journal, ,
no abstracts in English
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
no journal, ,
Adsorption behaviors of trivalent actinides (An(III)) and lanthanides (Ln(III)) to several activated carbons from nitric acid were investigated. Adsorptions of both An(III) and Ln(III) to carbon materials increased with an increase of pH of aqueous phase. In addition, it was found that An(III) was selectively adsorbed to activated carbons, compared with Ln(III). In column experiment using Am(III) and Eu(III), each elution peak was observed separately. It was found out that the use of activated carbon was effective for selective separation of An(III).
Watanabe, Masayuki; Arisaka, Makoto; Kimura, Takaumi
no journal, ,
In this presentation, new approach to separation of actinides from lanthanides with selective adsorption of trivalent actinides by using of carbon materials such as activated carbon, graphite or carbon nanotubes. Generally surface of activated carbon was tereated with concentrated acid to generate oxidized functional groups, such as carboxylate or phenolate, when activated carbon was used for adsorption of metal cations. Recentry, we have found that carbon materials was effective for separation without any pre-treatment. In this presentation, we would report the adsorption behavior of trivalent actinides on graphite and carbon nanotubes as well as columun chromatograph by using carbon materials.
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
no journal, ,
no abstracts in English
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
no journal, ,
For recovery of metal ions from a solution, pretreatment by oxidant acids was usually performed to introduce functional groups onto the surface of carbon materials because the adsorption of metal ions are mainly controlled by electrostatic forces, which are related to the various surface functional groups. In this study, adsorption behaviors of trivalent actinides (An(III)) and lanthanides (Ln(III)) to several carbon materials, such as carbon nanotubes, carbon powder and activated carbon, from acid solutions were investigated without such pretreatment. Both An(III) and Ln(III) was adsorbed strongly to carbon materials in the range from pH 1 to 4. In addition, it was found that An(III) was selectively adsorbed to carbon materials, compared with Ln(III).
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
no journal, ,
Adsorption behaviors of trivalent actinides (An(III)) and lanthanides (Ln(III)) to activated carbons with and without oxidation by concentrated nitric acid were investigated. Both An(III) and Ln(III) was adsorbed to activated carbons in the range from pH 1 to 4, and the distribution coefficients (
 ) of An(III) and Ln(III) increase with an increase of pH. Activated carbon without oxidation exhibits significant selectivity to An(III) compared with Ln(III). On the other hand, the oxidation to activated carbon deteriorates the selectivity to An(III). The depletion by the oxidation was attributed to the increase of functional groups of oxygen-donor, e.g., carboxyl and/or carbonyl groups because these functional groups are unfavorable for separation between An(III) and Ln(III). The selectivity of activated carbon without oxidation would be attributed to the interaction between
) of An(III) and Ln(III) increase with an increase of pH. Activated carbon without oxidation exhibits significant selectivity to An(III) compared with Ln(III). On the other hand, the oxidation to activated carbon deteriorates the selectivity to An(III). The depletion by the oxidation was attributed to the increase of functional groups of oxygen-donor, e.g., carboxyl and/or carbonyl groups because these functional groups are unfavorable for separation between An(III) and Ln(III). The selectivity of activated carbon without oxidation would be attributed to the interaction between  -electrons of aromatic ring and the metal ions.
-electrons of aromatic ring and the metal ions.