Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
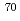 Se from combined conversion-electron and
Se from combined conversion-electron and  -ray spectroscopy
-ray spectroscopySmallcombe, J.; Garnsworthy, A. B.*; Korten, W.*; Singh, P.*; Muir, D.*; Pr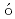 chniak, L.*; Ali, F. A.*; Andreoiu, C.*; Ansari, S.*; Ball, G. C.*; et al.
chniak, L.*; Ali, F. A.*; Andreoiu, C.*; Ansari, S.*; Ball, G. C.*; et al.
Physical Review C, 110(2), p.024318_1 - 024318_16, 2024/08
Times Cited Count:1 Percentile:52.60(Physics, Nuclear)Massey, D.*; Williams, C. D.*; Mu, J.*; Masters, A. J.*; Motokawa, Ryuhei; Aoyagi, Noboru; Ueda, Yuki; Antonio, M. R.*
Journal of Physical Chemistry B, 127(9), p.2052 - 2065, 2023/03
Times Cited Count:2 Percentile:14.56(Chemistry, Physical)Yakushev, A.*; Lens, L.*; D llmann, Ch. E.*; Khuyagbaatar, J.*; J
llmann, Ch. E.*; Khuyagbaatar, J.*; J ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
Frontiers in Chemistry (Internet), 10, p.976635_1 - 976635_11, 2022/08
Times Cited Count:19 Percentile:79.78(Chemistry, Multidisciplinary)Flerovium (Fl, element 114) is the heaviest element chemically studied so far. The first chemical experiment on Fl suggested that Fl is a noble-gas-like element, while the second studies suggested that Fl has a volatile-metal-like character. To obtain more reliable conclusion, we performed further experimental studies on Fl adsorption behavior on Si oxide and gold surfaces. The present results suggest that Fl is highly volatile and less reactive than the volatile metal, Hg, but has higher reactivity than the noble gas, Rn.
Do, S.-H.*; Paddison, J. A. M.*; Sala, G.*; Williams, T. J.*; Kaneko, Koji; Kuwahara, Keitaro*; May, A. F.*; Yan, J.*; McGuire, M. A.*; Stone, M. B.*; et al.
Physical Review B, 106(6), p.L060408_1 - L060408_6, 2022/08
Times Cited Count:16 Percentile:80.27(Materials Science, Multidisciplinary)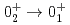
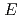 0 transition strength for
0 transition strength for 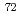 Se using the SPICE spectrometer
Se using the SPICE spectrometerSmallcombe, J.; Garnsworthy, A. B.*; Korten, W.*; Singh, P.*; Ali, F. A.*; Andreoiu, C.*; Ansari, S.*; Ball, G. C.*; Barton, C. J.*; Bhattacharjee, S. S.*; et al.
Physical Review C, 106(1), p.014312_1 - 014312_9, 2022/07
Times Cited Count:5 Percentile:60.14(Physics, Nuclear)Zarazovski, M.*; Pistra, V.*; Lauerova, D.*; Obermeier, F.*; Mora, D.*; Dubyk, Y.*; Bolinder, T.*; Cueto-Felgueroso, C.*; Szavai, S.*; Dudra, J.*; et al.
Proceedings of ASME 2022 Pressure Vessels and Piping Conference (PVP 2022) (Internet), 11 Pages, 2022/07
Motokawa, Ryuhei; Kobayashi, Toru; Endo, Hitoshi; Mu, J.*; Williams, C. D.*; Masters, A. J.*; Antonio, M. R.*; Heller, W. T.*; Nagao, Michihiro*
ACS Central Science, 5(1), p.85 - 96, 2019/01
Times Cited Count:56 Percentile:85.91(Chemistry, Multidisciplinary)Kristo, M. J.*; Williams, R.*; Gaffney, A. M.*; Kayzar-Boggs, T. M.*; Schorzman, K. C.*; Lagerkvist, P.*; Vesterlund, A.*; Rameb ck, H.*; Nelwamondo, A. N.*; Kotze, D.*; et al.
ck, H.*; Nelwamondo, A. N.*; Kotze, D.*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 315(2), p.425 - 434, 2018/02
Times Cited Count:17 Percentile:79.90(Chemistry, Analytical)In a recent international exercise, 10 international nuclear forensics laboratories successfully performed radiochronometry on three low enriched uranium oxide samples, providing 12 analytical results using three different parent-daughter pairs serving as independent chronometers. The vast majority of the results were consistent with one another and consistent with the known processing history of the materials. In general, for these particular samples, mass spectrometry gave more accurate and more precise analytical results than decay counting measurements. In addition, the concordance of the  U-
U-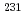 Pa and
Pa and  U-
U-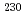 Th chronometers confirmed the validity of the age dating assumptions, increasing confidence in the resulting conclusions.
Th chronometers confirmed the validity of the age dating assumptions, increasing confidence in the resulting conclusions.
 -butyl phosphate in organic and aqueous environments and its relevance to nuclear extraction processes
-butyl phosphate in organic and aqueous environments and its relevance to nuclear extraction processesMu, J.*; Motokawa, Ryuhei; Williams, C. D.*; Akutsu, Kazuhiro*; Nishitsuji, Shotaro*; Masters, A. J.*
Journal of Physical Chemistry B, 120(23), p.5183 - 5193, 2016/06
Times Cited Count:27 Percentile:56.40(Chemistry, Physical)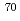 Ni
NiChiara, C. J.*; Weisshaar, D.*; Janssens, R. V. F.*; Tsunoda, Yusuke*; Otsuka, Takaharu*; Harker, J. L.*; Walters, W. B.*; Recchia, F.*; Albers, M.*; Alcorta, M.*; et al.
Physical Review C, 91(4), p.044309_1 - 044309_10, 2015/04
Times Cited Count:40 Percentile:90.62(Physics, Nuclear)The neutron-rich isotope 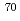 Ni was produced by multi-nucleon transfer reactions of
Ni was produced by multi-nucleon transfer reactions of 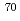 Zn in the Argonne National Laboratory, and an in-beam
Zn in the Argonne National Laboratory, and an in-beam  -ray experiment were performed using the GRETINA array. The
-ray experiment were performed using the GRETINA array. The 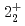 and
and 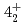 levels of
levels of 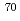 Ni were observed for the first time. Those levels are regarded as large deformed states associated with proton excitation from the
Ni were observed for the first time. Those levels are regarded as large deformed states associated with proton excitation from the 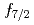 orbit because they cannot be reproduced by a shell-model calculation assuming a small valence space without
orbit because they cannot be reproduced by a shell-model calculation assuming a small valence space without 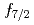 . A theoretical analysis based on the Monte Carlo shell model published in 2014 indicates that those levels corresponds to a prolate deformed band. The present result demonstrates the occurrence of shape coexistence in neutron-rich Ni isotopes other than a known case of
. A theoretical analysis based on the Monte Carlo shell model published in 2014 indicates that those levels corresponds to a prolate deformed band. The present result demonstrates the occurrence of shape coexistence in neutron-rich Ni isotopes other than a known case of 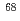 Ni, and confirms the predictive power of the Monte Carlo shell-model calculation.
Ni, and confirms the predictive power of the Monte Carlo shell-model calculation.
Deng, Z.*; Jin, C. Q.*; Liu, Q. Q.*; Wang, X. C.*; Zhu, J. L.*; Feng, S. M.*; Chen, L. C.*; Yu, R. C.*; Arguello, C.*; Goko, Tatsuo*; et al.
Nature Communications (Internet), 2, p.1425_1 - 1425_5, 2011/08
Times Cited Count:172 Percentile:93.60(Multidisciplinary Sciences)In a prototypical ferromagnet (Ga,Mn)As based on a III-V semiconductor, substitution of divalent Mn atoms into trivalent Ga sites leads to severely limited chemical solubility and metastable specimens available only as thin films. The doping of hole carriers via (Ga,Mn) substitution also prohibits electron doping. To overcome these difficulties, Masek et al. theoretically proposed systems based on a I-II-V semiconductor LiZnAs, where isovalent (Zn,Mn) substitution is decoupled from carrier doping with excess/deficient Li concentrations. Here we show successful synthesis of Li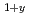 (Zn
(Zn Mn
Mn )As in bulk materials. We reported that ferromagnetism with a critical temperature of up to 50 K is observed in nominally Li-excess compounds, which have p-type carriers.
)As in bulk materials. We reported that ferromagnetism with a critical temperature of up to 50 K is observed in nominally Li-excess compounds, which have p-type carriers.
 Se nuclear shapes with SPICE and TIGRESS
Se nuclear shapes with SPICE and TIGRESSSmallcombe, J.; Garnsworthy, A. B.*; Korten, W.*; Singh, P.*; Ali, F. A.*; Andreoiu, C.*; Ansari, S.*; Ball, G. C.*; Barton, C. J.*; Bhattacharjee, S. S.*; et al.
no journal, ,