Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 (001) surface using supersonic seeded oxygen molecular beam
(001) surface using supersonic seeded oxygen molecular beamKatsube, Daiki*; Ono, Shinya*; Takayanagi, Shuhei*; Ojima, Shoki*; Maeda, Motoyasu*; Origuchi, Naoki*; Ogawa, Arata*; Ikeda, Natsuki*; Aoyagi, Yoshihide*; Kabutoya, Yuito*; et al.
Langmuir, 37(42), p.12313 - 12317, 2021/10
Times Cited Count:1 Percentile:6.39(Chemistry, Multidisciplinary)We investigated the oxidation of oxygen vacancies at the surface of anatase TiO (001) using supersonic seeded molecular beam (SSMB) of oxygen. The oxygen vacancies at the top-surface and sub-surface could be eliminated by the supply of oxygen using an SSMB. These results indicate that the interstitial vacancies can be mostly assigned to oxygen vacancies, which can be effectively eliminated by using an oxygen SSMB. Oxygen vacancies are present on the surface of anatase TiO
(001) using supersonic seeded molecular beam (SSMB) of oxygen. The oxygen vacancies at the top-surface and sub-surface could be eliminated by the supply of oxygen using an SSMB. These results indicate that the interstitial vacancies can be mostly assigned to oxygen vacancies, which can be effectively eliminated by using an oxygen SSMB. Oxygen vacancies are present on the surface of anatase TiO (001) when it is untreated before transfer to a vacuum chamber. These vacancies, which are stable in the as-grown condition, could also be effectively eliminated using the oxygen SSMB.
(001) when it is untreated before transfer to a vacuum chamber. These vacancies, which are stable in the as-grown condition, could also be effectively eliminated using the oxygen SSMB.
Sano, Asami; Ito, Shoichi*; Suzumura, Akimasa*; Ueno, Yuichiro*; Yagi, Hikaru*; Inoue, Toru*; Kawazoe, Takaaki*
Koatsuryoku No Kagaku To Gijutsu, 30(2), p.85 - 94, 2020/10
Minerals and rocks exhibit various isotope compositions depending on their origins and histories. In interpreting their isotopic variations, the equilibrium isotope fractionation factor is a key because it depends on the environment parameters such as temperature. Recent studies have shown that the effect of pressure on the isotope fractionation, which was considered negligible compared to temperature, is significant under the conditions of the Earth's interior. In this article we review recent advances in experimental studies to determine the isotope fractionation of iron and hydrogen at high pressure over several GPa, discussing their issues and future perspectives.
Yoneda, Yasuhiro; Mizuki, Junichiro; Katayama, Ryoko*; Yagi, Kenichiro*; Terauchi, Hikaru*; Hamazaki, Shinichi*; Takashige, Masaaki*
Applied Physics Letters, 83(2), p.275 - 277, 2003/07
Times Cited Count:18 Percentile:57.3(Physics, Applied)We observed an intermediate structure during the re-crystallization process from the amorphous state of Bi Ti
Ti O
O prepared by rapid quenching. The intermediate structure which appears during the re-crystallization process consists of two phases; one is pyrochlore Bi
prepared by rapid quenching. The intermediate structure which appears during the re-crystallization process consists of two phases; one is pyrochlore Bi Ti
Ti O
O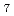 phase and the other is a stacking-fault induced structure under the excessive Bi condition. The microstructure of the stacking-fault induced structure was investigated by synchrotron X-ray diffraction. In the case of a large number of Bi
phase and the other is a stacking-fault induced structure under the excessive Bi condition. The microstructure of the stacking-fault induced structure was investigated by synchrotron X-ray diffraction. In the case of a large number of Bi O
O , some are inserted between the pseudo-perovskite layers of Bi
, some are inserted between the pseudo-perovskite layers of Bi Ti
Ti O
O , and a non-stoichiometric Bi
, and a non-stoichiometric Bi WO
WO -like structure is stabilized.
-like structure is stabilized.
 Fe
Fe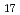 Nx
NxKasatani, Hirofumi*; Omura, Masashi*; Yokoyama, Katsumi*; Kobayashi, Kurima*; Nishihata, Yasuo; Yagi, Kenichiro*; Terauchi, Hikaru*
Japanese Journal of Applied Physics, 38(Suppl.38-1), p.433 - 435, 1999/06
Times Cited Count:3 Percentile:18.86(Physics, Applied)High energy XAFS studies of Sm: K-edge in Sm2Fe17Nx with x = 0.0, <= 0.1, 0.5, 2.0 and 3.0 were carried out at room temperature in order to clarify the relation between the local structure around the Sm-atom and the change of the magnetic property due to the absorption of nitrogen atom. In the XANES region, we firstly found the change of Sm electron state by nitriding because of the change of the shape in the absorption edge junp. In the result of the EXAFS spectra, we confirmed that the Sm-Fe distance was expanded monotonously due to the absorption of N-atom.
Tsukada, Chie; Yoshida, Hikaru; Ogawa, Satoshi*; Yoshigoe, Akitaka; Yagi, Shinya*; Yaita, Tsuyoshi
no journal, ,
We focus on the Au nanoparticle, which is fabricated by solution plasma method, as the adsorbent with high density and high efficiency of Cs in solution. To remove the Cs from the solution, we attempt two methods, which are the Cs adsorption on the Au nanoparticle surface and the interaction between the Cs and the L-cysteine on Au nanoparticle. Judging from the results of SR-XPS for the sample at the latter method, we suggest the interaction between the Cs and the COOH group of L-cysteine on Au nanoparticle.
in solution. To remove the Cs from the solution, we attempt two methods, which are the Cs adsorption on the Au nanoparticle surface and the interaction between the Cs and the L-cysteine on Au nanoparticle. Judging from the results of SR-XPS for the sample at the latter method, we suggest the interaction between the Cs and the COOH group of L-cysteine on Au nanoparticle.
Tsukada, Chie*; Yoshida, Hikaru; Ogawa, Satoshi*; Yoshigoe, Akitaka; Yagi, Shinya*; Yaita, Tsuyoshi
no journal, ,
The decontamination of radioactive Cs from the soil and the water has been required in Fukushima. Gold nanoparticles (AuNPs) are attractive candidate for Cs adsorbent. AuNPs fabricated by solution plasma method is not covered by dispersant and is almost clean. It is known that L-cysteine rapidly adsorbs and exists with high density on the AuNPs surface. The electrostatic attractive force may be useful to induce interactions between -COO- of L-cysteine and Cs ion in aqueous solution. This study reveals the adsorption reaction between the Cs and the Cys/AuNP. Cys/AuNP has a peak and CsCl/Cys/AuNP has no peak in Na 1s spectra. Cys/AuNP has no peak and CsCl/Cys/AuNP has a peak for Cs 3
ion in aqueous solution. This study reveals the adsorption reaction between the Cs and the Cys/AuNP. Cys/AuNP has a peak and CsCl/Cys/AuNP has no peak in Na 1s spectra. Cys/AuNP has no peak and CsCl/Cys/AuNP has a peak for Cs 3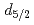 spectra. From these results, Na
spectra. From these results, Na on Cys/AuNP is replaced by Cs
on Cys/AuNP is replaced by Cs after reaction in CsCl aqueous solution. Cys/AuNP and CsCl/Cys/AuNP have a O 1s peak, where the peak position of CsCl/Cys/AuNP is higher than that ofCys/AuNP. This indicates the further polarization of -COO- by replacing Na
after reaction in CsCl aqueous solution. Cys/AuNP and CsCl/Cys/AuNP have a O 1s peak, where the peak position of CsCl/Cys/AuNP is higher than that ofCys/AuNP. This indicates the further polarization of -COO- by replacing Na by Cs
by Cs .
.
Tsukada, Chie*; Yoshida, Hikaru; Ogawa, Satoshi*; Yoshigoe, Akitaka; Yagi, Shinya*; Yaita, Tsuyoshi
no journal, ,
To recovery from the Fukushima Daiichi Nuclear Power Station accident, the removal of radioactive Cs from the soil and cooling water is important. An absorbent and a removal procedure of Cs are required to have properties of effective separation ability, recyclable and energy saving. Here, we focus on the gold nanoparticles (AuNPs) fabricated by solution plasma method. The purpose of this study is to reveal the surface chemical states of the AuNPs fabricated by solution plasma method in CsCl aqueous solution. From the synchrotron light based analysis, it was found that the Cs-Cl-Au bondings form by the repeat of below two steps. (1) Cs and Cl atoms adsorb on AuNPs surface (Cs-Au and Cl-Au bondings). (2) Au atoms bonds around the AuNPs surface.