Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:0no abstracts in English
Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:1 Percentile:51.1(Chemistry, Multidisciplinary)no abstracts in English
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Tsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru; Yaita, Tsuyoshi
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(1), p.15 - 18, 2023/02
no abstracts in English
Honda, Mitsunori; Kaneta, Yui; Yaita, Tsuyoshi
AIP Advances (Internet), 13(1), p.015314_1 - 015314_6, 2023/01
Times Cited Count:0 Percentile:0(Nanoscience & Nanotechnology)The efficiency of the sorption of Sr on weathered biotite, a type of clay minerals was investigated. Removal of Sr
on weathered biotite, a type of clay minerals was investigated. Removal of Sr and Cs
and Cs is important in the treatment of contaminated water from the 1F accident, which is one of the radionuclide waste treatment problems. We focused on developing an adsorption method for Sr ions using weathered biotite, which are abundant in Fukushima. Applying a molten salt treatment, the amount of sorbed Sr
is important in the treatment of contaminated water from the 1F accident, which is one of the radionuclide waste treatment problems. We focused on developing an adsorption method for Sr ions using weathered biotite, which are abundant in Fukushima. Applying a molten salt treatment, the amount of sorbed Sr simply increased as the added mass ratio of strontium chloride (SrCl
simply increased as the added mass ratio of strontium chloride (SrCl ) increased from 1:1, 1:5, and 1:10 for the one-fold, five-fold, and ten-fold additions of SrCl
) increased from 1:1, 1:5, and 1:10 for the one-fold, five-fold, and ten-fold additions of SrCl , respectively. Then, the crystal structure of weathered biotite as an adsorbent was evaluated by X-ray diffraction (XRD) analysis. Thus, it was observed that the WB retained its original crystal structure even after the sorption of Sr
, respectively. Then, the crystal structure of weathered biotite as an adsorbent was evaluated by X-ray diffraction (XRD) analysis. Thus, it was observed that the WB retained its original crystal structure even after the sorption of Sr . Extended X-ray absorption fine structure (EXAFS) analysis was performed to investigate the local sorption structure of Sr
. Extended X-ray absorption fine structure (EXAFS) analysis was performed to investigate the local sorption structure of Sr in the WB. The results revealed that Sr
in the WB. The results revealed that Sr was preferentially sorbed into the SiO
was preferentially sorbed into the SiO and Al
and Al O
O layers when Sr
layers when Sr was in the low mass ratio, while, it was mainly sorbed into the SiO
was in the low mass ratio, while, it was mainly sorbed into the SiO layer when the ratio was high.
layer when the ratio was high.
 Np and
Np and  Am gamma ray regions
Am gamma ray regionsFukuda, Tatsuo; Kobata, Masaaki; Shobu, Takahisa; Yoshii, Kenji; Kamiya, Junichiro; Iwamoto, Yosuke; Makino, Takahiro*; Yamazaki, Yuichi*; Oshima, Takeshi*; Shirai, Yasuhiro*; et al.
Journal of Applied Physics, 132(24), p.245102_1 - 245102_8, 2022/12
Times Cited Count:1 Percentile:17.38(Physics, Applied)Direct energy conversion has been investigated using Ni/SiC Schottky junctions with the irradiation of monochromatized synchrotron X-rays simulating the gamma rays of  Np (30 keV) and
Np (30 keV) and  Am (60 keV). From current-voltage measurements, electrical energies were obtained for both kinds of gamma rays. The energy conversion efficiencies were found to reach up to
Am (60 keV). From current-voltage measurements, electrical energies were obtained for both kinds of gamma rays. The energy conversion efficiencies were found to reach up to  1.6%, which is comparable to those of a few other semiconducting systems reported thus far. This result shows a possibility of energy recovery from nuclear wastes using the present system, judging from the radiation tolerant nature of SiC. Also, we found different conversion efficiencies between the two samples. This could be understandable from hard X-ray photoelectron spectroscopy and secondary ion mass spectroscopy measurements, suggesting the formation of Ni-Si compounds at the interface in the sample with a poor performance. Hence, such combined measurements are useful to provide information that cannot be obtained by electrical measurements alone.
1.6%, which is comparable to those of a few other semiconducting systems reported thus far. This result shows a possibility of energy recovery from nuclear wastes using the present system, judging from the radiation tolerant nature of SiC. Also, we found different conversion efficiencies between the two samples. This could be understandable from hard X-ray photoelectron spectroscopy and secondary ion mass spectroscopy measurements, suggesting the formation of Ni-Si compounds at the interface in the sample with a poor performance. Hence, such combined measurements are useful to provide information that cannot be obtained by electrical measurements alone.
 molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:9 Percentile:68.46(Multidisciplinary Sciences)no abstracts in English
Shimojo, Kojiro; Fujiwara, Iori*; Oshima, Tatsuya*; Yokoyama, Keiichi; Yaita, Tsuyoshi
Analytical Sciences, 38(7), p.1003 - 1006, 2022/07
Times Cited Count:0 Percentile:0(Chemistry, Analytical)Liquid-liquid extraction of lanthanide (Ln) ions was investigated using  -dioctylthiodiglycolamic acid (DOTDGAA), which is a sulfur donor ligand with an amide group and a carboxyl group connected by a thioether chain. The extraction performance and selectivity of DOTDGAA for Ln ions were compared with those of
-dioctylthiodiglycolamic acid (DOTDGAA), which is a sulfur donor ligand with an amide group and a carboxyl group connected by a thioether chain. The extraction performance and selectivity of DOTDGAA for Ln ions were compared with those of  -dioctyldiglycolamic acid (DODGAA), which is also an oxygen donor ligand with a similar chemical structure, to assess the effect of the soft/hard donor atom on Ln separation. DOTDGAA quantitatively extracted all Ln ions while being selective toward light and middle Ln ions, in contrast to the selectivity of DODGAA for heavier Ln ions. Slope analysis demonstrated that the Ln
-dioctyldiglycolamic acid (DODGAA), which is also an oxygen donor ligand with a similar chemical structure, to assess the effect of the soft/hard donor atom on Ln separation. DOTDGAA quantitatively extracted all Ln ions while being selective toward light and middle Ln ions, in contrast to the selectivity of DODGAA for heavier Ln ions. Slope analysis demonstrated that the Ln transfer using DOTDGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, Ln(DOTDGAA)
transfer using DOTDGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, Ln(DOTDGAA) . The back-extraction of Ln ions from the extracting phase was successfully achieved under acidic conditions.
. The back-extraction of Ln ions from the extracting phase was successfully achieved under acidic conditions.
Sekiguchi, Tetsuhiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
e-Journal of Surface Science and Nanotechnology (Internet), 20(3), p.186 - 195, 2022/07
Cesium-135 having long life, 2.3 million y, that is contained in nuclear wastes may cause long-term pollution. Technology of isotopic separation of such long lived nuclide is indispensable not only for its volume reduction but also annihilation by nuclear transmutation. The recovery of atomic Cs from molecular CsI is mandatory. We have investigated fullerene C as a potential absorber for Cs. Angle-resolved X-ray photoelectron spectroscopy, AR-XPS has been used to analyze the depth concentration distribution of Cs. Experiments were performed at soft X-ray beamline BL27A at KEK PF facility. We report on the annealing effect after deposition of Cs and the effect of heating substrate during deposition. For Cs/C
as a potential absorber for Cs. Angle-resolved X-ray photoelectron spectroscopy, AR-XPS has been used to analyze the depth concentration distribution of Cs. Experiments were performed at soft X-ray beamline BL27A at KEK PF facility. We report on the annealing effect after deposition of Cs and the effect of heating substrate during deposition. For Cs/C sample, the intensity ratio of Cs-3d/C-1s increased in double at the high temperature. This suggests that Cs atoms remain in the material at high temperatures. On the other hand, for CsI/C
sample, the intensity ratio of Cs-3d/C-1s increased in double at the high temperature. This suggests that Cs atoms remain in the material at high temperatures. On the other hand, for CsI/C , the intensity ratio does not change much by elevating temperatures.
, the intensity ratio does not change much by elevating temperatures.
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:6 Percentile:58.16(Multidisciplinary Sciences)no abstracts in English
Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:21.56(Chemistry, Inorganic & Nuclear)no abstracts in English
Simonnet, M.; Kobayashi, Toru; Shimojo, Kojiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
Inorganic Chemistry, 60(17), p.13409 - 13418, 2021/09
Times Cited Count:15 Percentile:87.32(Chemistry, Inorganic & Nuclear)Shimojo, Kojiro; Suzuki, Hideya; Yokoyama, Keiichi; Yaita, Tsuyoshi; Ikeda, Atsushi
Analytical Sciences, 36(12), p.1435 - 1437, 2020/12
Times Cited Count:13 Percentile:64.65(Chemistry, Analytical)Liquid-liquid extraction for the removal of pertechnetate ( TcO
TcO
 ) and perrhenate (ReO
) and perrhenate (ReO
 ) is reported using tripodal extractant
) is reported using tripodal extractant 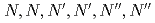 -hexa-
-hexa- -octylnitrilotriacetamide (HONTA) composed of three amide groups and a tertiary amine. The extraction behavior was compared with those using alkyldiamideamines (ADAAM(Oct) and ADAAM(EH)), and the commercial amine-type extractant, trioctylamine (TOA). HONTA quantitatively extracted
-octylnitrilotriacetamide (HONTA) composed of three amide groups and a tertiary amine. The extraction behavior was compared with those using alkyldiamideamines (ADAAM(Oct) and ADAAM(EH)), and the commercial amine-type extractant, trioctylamine (TOA). HONTA quantitatively extracted  TcO
TcO
 and ReO
and ReO
 in the pH range from 1.0 to 2.5 by co-extraction of protons. Extraction performance of extractants was improved in the order of HONTA
in the pH range from 1.0 to 2.5 by co-extraction of protons. Extraction performance of extractants was improved in the order of HONTA  ADAAM(Oct)
ADAAM(Oct)  ADAAM(EH)
ADAAM(EH)  TOA.
TOA.  TcO
TcO
 and ReO
and ReO
 in the extracting phase were successfully stripped using neutral aqueous solutions as the receiving phase, and the extraction ability of HONTA was maintained after five repeated uses.
in the extracting phase were successfully stripped using neutral aqueous solutions as the receiving phase, and the extraction ability of HONTA was maintained after five repeated uses.
Orlandi, R.; Hirose, Kentaro; Yaita, Tsuyoshi; Yamagami, Hiroshi; Ieda, Junichi; Kambe, Shinsaku; Ishikawa, Norito
Nihon Genshiryoku Gakkai-Shi ATOMO , 62(5), p.280 - 284, 2020/05
, 62(5), p.280 - 284, 2020/05
no abstracts in English
 Fe
Fe O
O
Kobata, Masaaki; Yoshii, Kenji; Fukuda, Tatsuo; Kawasaki, Ikuto; Okane, Tetsuo; Yamagami, Hiroshi; Yaita, Tsuyoshi; Harii, Kazuya; Ieda, Junichi; Okayasu, Satoru; et al.
JPS Conference Proceedings (Internet), 30, p.011192_1 - 011192_6, 2020/03
High energy X-ray photoelectron spectroscopy (HAXPES) measurements were carried out for the Spin Seebeck system Pt/Y Fe
Fe O
O (YIG). This system was found to show anomalous Hall effect, possible due to the formation of intermetallic compounds between Fe
(YIG). This system was found to show anomalous Hall effect, possible due to the formation of intermetallic compounds between Fe and Pt. To reveal this possibility, we have measured the Fe 1s photoelectron peaks by using HAXPES. It was found that the Fe ions consist of Fe
and Pt. To reveal this possibility, we have measured the Fe 1s photoelectron peaks by using HAXPES. It was found that the Fe ions consist of Fe in YIG and metallic Fe. The formation of the metallic state is consistent with the proposed origin of the anomalous Hall effect. Other spectra such as Pt 4f will be presented at the conference.
in YIG and metallic Fe. The formation of the metallic state is consistent with the proposed origin of the anomalous Hall effect. Other spectra such as Pt 4f will be presented at the conference.
Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:18 Percentile:69.88(Chemistry, Multidisciplinary)Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:3 Percentile:12.45(Chemistry, Analytical)no abstracts in English
Narita, Hirokazu*; Nicolson, R. M.*; Motokawa, Ryuhei; Ito, Fumiyuki*; Morisaku, Kazuko*; Goto, Midori*; Tanaka, Mikiya*; Heller, W. T.*; Shiwaku, Hideaki; Yaita, Tsuyoshi; et al.
Inorganic Chemistry, 58(13), p.8720 - 8734, 2019/07
Times Cited Count:14 Percentile:69.69(Chemistry, Inorganic & Nuclear) -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:14.02(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.