Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Chernozatonskii, L. A.*; Sorokin, P. B.*; Kuzubov, A. A.*; Sorokin, B. P.*; Kvashnin, A. G.*; Kvashnin, D. G.*; Avramov, P.; Yakobson, B. I.*
Journal of Physical Chemistry C, 115(1), p.132 - 136, 2011/01
Times Cited Count:80 Percentile:88.67(Chemistry, Physical)Avramov, P.; Yakobson, B. I.*
Journal of Physical Chemistry A, 111(8), p.1508 - 1514, 2007/03
Times Cited Count:7 Percentile:23.94(Chemistry, Physical)The mechanism of interaction of low-energy atoms and ions of light elements (H, H , He, Li, the kinetic energy of the particles 2-40 eV) with C
, He, Li, the kinetic energy of the particles 2-40 eV) with C H
H , C
, C F
F , C
, C , and C
, and C F
F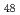 molecules was studied by ab initio MD simulations and quantum chemical calculations. It was shown that for the "C
molecules was studied by ab initio MD simulations and quantum chemical calculations. It was shown that for the "C H
H + proton" and "C
+ proton" and "C + proton" systems, the electronic charge transfer from the aromatic molecule to H
+ proton" systems, the electronic charge transfer from the aromatic molecule to H occurs with a probability close to 1. The process transforms the H
occurs with a probability close to 1. The process transforms the H to a hydrogen atom and the neutral C
to a hydrogen atom and the neutral C H
H and C
and C molecules to cation radicals. The mechanism of interaction of low-energy protons with C
molecules to cation radicals. The mechanism of interaction of low-energy protons with C F
F and C
and C F
F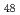 molecules has a substantially different character and can be considered qualitatively as the interaction between a neutral molecule and a point charge. The Coulomb perturbation of the system arising from the interaction of the uncompensated proton charge with the Mulliken charges of fluorine atoms results in an inversion of the energies of the electronic states localized on the proton and on the C
molecules has a substantially different character and can be considered qualitatively as the interaction between a neutral molecule and a point charge. The Coulomb perturbation of the system arising from the interaction of the uncompensated proton charge with the Mulliken charges of fluorine atoms results in an inversion of the energies of the electronic states localized on the proton and on the C F
F and C
and C F
F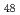 molecules and makes the electronic charge transfer energetically unfavorable. The barriers of the proton penetration for the C
molecules and makes the electronic charge transfer energetically unfavorable. The barriers of the proton penetration for the C F
F and C
and C F
F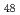 molecules are from two to four times lower than for the corresponding parent systems (C
molecules are from two to four times lower than for the corresponding parent systems (C H
H and C
and C ). The penetration barriers of the He atom and Li
). The penetration barriers of the He atom and Li ion depend mainly on the effective radii of the bombarding particles.
ion depend mainly on the effective radii of the bombarding particles.