Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamamoto, Kazami; Moriya, Katsuhiro; Okita, Hidefumi; Yamada, Ippei; Chimura, Motoki; Saha, P. K.; Shobuda, Yoshihiro; Tamura, Fumihiko; Yamamoto, Masanobu; Morishita, Takatoshi; et al.
Journal of Neutron Research, 26(2-3), p.59 - 67, 2024/01
The linac and 3 GeV rapid cycling synchrotron at the Japan Proton Accelerator Research Complex was designed to provide 1-MW proton beams to the following facilities. Thanks to the improvement works of the accelerator system, we successfully accelerate 1-MW beam with quite small beam loss. Currently, the beam power of RCS is limited by the lack of anode current in the RF cavity system rather than the beam loss. Recently we developed a new acceleration cavity that can accelerate a beam with less anode current. This new cavity enables us not only to reduce requirement of the anode power supply but also to accelerate more than 1-MW beam. We have started to consider the way to achieve beyond 1-MW beam acceleration. So far, it is expected that up to 1.5-MW beam can be accelerated after replacement of the RF cavity. We have also been continuing study to achieve up to 2 MW beam in J-PARC RCS.
Yamamoto, Kazami; Moriya, Katsuhiro; Okita, Hidefumi; Yamada, Ippei; Chimura, Motoki; Saha, P. K.; Shobuda, Yoshihiro; Tamura, Fumihiko; Yamamoto, Masanobu; Morishita, Takatoshi; et al.
Proceedings of 68th ICFA Advanced Beam Dynamics Workshop on High Intensity and High Brightness Hadron Beams (HB2023) (Internet), p.270 - 273, 2023/10
The 3-GeV rapid-cycling synchrotron at the Japan Pro-ton Accelerator Research Complex was designed to provide 1-MW proton beams to the following facilities. Thanks to the improvement works of the accelerator system, we successfully accelerate 1-MW beam with quite small beam loss. Currently, the beam power of RCS is limited by the lack of anode current in the RF cavity system rather than the beam loss. Recently we developed a new acceleration cavity that can accelerate a beam with less anode current. This new cavity enables us not only to reduce requirement of the anode power supply but also to accelerate more than 1-MW beam. We have started to consider the way to achieve beyond 1-MW beam acceleration. So far, it is expected that up to 1.5-MW beam can be accelerated after replacement of the RF cavity. We have also continued study to achieve more than 2 MW beam in J-PARC RCS.
Ogawa, Shuichi*; Tsuda, Yasutaka; Sakamoto, Tetsuya*; Okigawa, Yuki*; Masuzawa, Tomoaki*; Yoshigoe, Akitaka; Abukawa, Tadashi*; Yamada, Takatoshi*
Applied Surface Science, 605, p.154748_1 - 154748_6, 2022/12
Times Cited Count:7 Percentile:47.60(Chemistry, Physical)Immersion of graphene in KOH solution improves its mobility on SiO /Si wafers. This is thought to be due to electron doping by modification with K atoms, but the K atom concentration C
/Si wafers. This is thought to be due to electron doping by modification with K atoms, but the K atom concentration C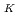 in the graphene has not been clarified yet. In this study, the C
in the graphene has not been clarified yet. In this study, the C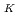 was determined by XPS analysis using high-brilliance synchrotron radiation. The time evolution of C
was determined by XPS analysis using high-brilliance synchrotron radiation. The time evolution of C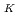 was determined by real-time observation, and the C
was determined by real-time observation, and the C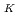 before irradiation of synchrotron radiation was estimated to be 0.94%. The C 1s spectrum shifted to the low binding energy side with the desorption of K atoms. This indicates that the electron doping concentration into graphene is decreasing, and it is experimentally confirmed that K atoms inject electrons into graphene.
before irradiation of synchrotron radiation was estimated to be 0.94%. The C 1s spectrum shifted to the low binding energy side with the desorption of K atoms. This indicates that the electron doping concentration into graphene is decreasing, and it is experimentally confirmed that K atoms inject electrons into graphene.
Ogawa, Shuichi*; Yamaguchi, Hisato*; Holby, E. F.*; Yamada, Takatoshi*; Yoshigoe, Akitaka; Takakuwa, Yuji*
Journal of Physical Chemistry Letters (Internet), 11(21), p.9159 - 9164, 2020/11
Times Cited Count:4 Percentile:18.74(Chemistry, Physical)Atomically thin layers of graphene have been proposed to protect surfaces through the direct blocking of corrosion reactants such as oxygen with low added weight. The long term efficacy of such an approach, however, is unclear due to the long-term desired protection of decades and the presence of defects in as-synthesized materials. Here, we demonstrate catalytic permeation of oxygen molecules through previously-described impermeable graphene by imparting sub-eV kinetic energy to molecules. These molecules represent a small fraction of a thermal distribution thus this exposure serves as an accelerated stress test for understanding decades-long exposures. The permeation rate of the energized molecules increased 2 orders of magnitude compared to their non-energized counterpart. Graphene maintained its relative impermeability to non-energized oxygen molecules even after the permeation of energized molecules indicating that the process is non-destructive and a fundamental property of the exposed material.
Yamaguchi, Hisato*; Ogawa, Shuichi*; Watanabe, Daiki*; Hozumi, Hideaki*; Gao, Y.*; Eda, Goki*; Mattevi, C.*; Fujita, Takeshi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; et al.
Physica Status Solidi (A), 213(9), p.2380 - 2386, 2016/09
Times Cited Count:14 Percentile:51.12(Materials Science, Multidisciplinary)We report valence-band electronic structure evolution of graphene oxide (GO) upon its thermal reduction. The degree of oxygen functionalization was controlled by annealing temperature, and an electronic structure evolution was monitored using real-time ultraviolet photoelectron spectroscopy. We observed a drastic increase in the density of states around the Fermi level upon thermal annealing at  600
600 C. The result indicates that while there is an apparent bandgap for GO prior to a thermal reduction, the gap closes after an annealing around that temperature. This trend of bandgap closure was correlated with the electrical, chemical, and structural properties to determine a set of GO material properties that is optimal for optoelectronics. The results revealed that annealing at a temperature of 500
C. The result indicates that while there is an apparent bandgap for GO prior to a thermal reduction, the gap closes after an annealing around that temperature. This trend of bandgap closure was correlated with the electrical, chemical, and structural properties to determine a set of GO material properties that is optimal for optoelectronics. The results revealed that annealing at a temperature of 500 C leads to the desired properties, demonstrated by a uniform and an order of magnitude enhanced photocurrent map of an individual GO sheet compared to an as-synthesized counterpart.
C leads to the desired properties, demonstrated by a uniform and an order of magnitude enhanced photocurrent map of an individual GO sheet compared to an as-synthesized counterpart.
 O
O substrates
substratesOgawa, Shuichi*; Yamada, Takatoshi*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
Japanese Journal of Applied Physics, 52(11), p.110122_1 - 110122_8, 2013/11
Times Cited Count:21 Percentile:62.72(Physics, Applied)Ogawa, Shuichi*; Yamada, Takatoshi*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
Japanese Journal of Applied Physics, 51(11), p.11PF02_1 - 11PF02_7, 2012/11
Times Cited Count:33 Percentile:74.78(Physics, Applied)Ogawa, Shuichi*; Yamada, Takatoshi*; Ishizuka, Shinji*; Watanabe, Daiki*; Yoshigoe, Akitaka; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
Hyomen Kagaku, 33(8), p.449 - 454, 2012/08
Graphene-on-insulator structures are required for fabrication of the graphene transistor. Diamond has been attracted as the substrate for graphene growth because it has a larger band gap and break down voltage compared with SiC. The detail of graphitization on a diamond surface has not been clarified yet because the nondestructive evaluation for graphene-on-diamond (GOD) structure was hard. In this study, we have developed an evaluation method of GOD based on the photoemission spectroscopy using synchrotron radiation focusing the shift of photoelectron spectra due to band bending. We can clearly determine the graphitization temperature on the diamond C(111) surface as approximately 1120 K, which is lower than that on an SiC substrate. It is also confirmed from C 1s photoelectron spectra, there is the buffer layer at the interface between the grapheme layer and the diamond substrate.
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
no abstracts in English
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
Ogawa, Shuichi*; Yamada, Takatoshi*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
no abstracts in English
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
no abstracts in English
Ogawa, Shuichi*; Yamada, Takatoshi*; Taga, Ryo*; Yoshigoe, Akitaka; Takakuwa, Yuji*
no journal, ,
The molecular adsorption on graphene causes the modulation of conductivity of graphene, so that the application of graphene for gas sensors are expected. To evaluate the type of conductivity of graphene by photoelectron spectroscopy (PES), the angle-resolved PES is widely used. However, ARPES measurements have been performed usually in synchrotron facility or using monochromatic ultraviolet light. In this study, we try to evaluate the valence band of graphene using angle-integrated PES using non-monochromatic He I line in order to evaluate the changes of valence band of graphene during adsorption and/or desorption of molecules. As summary, we can measure the changes of shift of Fermi level depending on the temperature by using filter based on Fourier transform. This change is caused by desorption of absorbed molecules on graphene, and we can follow up the change because of using high-intensity non-monochromatic He I resonance line.
 -FeSi
-FeSi homoepitaxial growth
homoepitaxial growthWakaya, Ippei*; Ochiai, Kunihito*; Udono, Haruhiko*; Nagano, Takatoshi*; Yamada, Yoichi; Yamamoto, Hiroyuki; Esaka, Fumitaka
no journal, ,
Deposit condition in the first stage of  -FeSi
-FeSi homoepitaxial growth has been investigated.
homoepitaxial growth has been investigated.
Sudo, Katsuo; Okita, Takatoshi; Takeuchi, Kentaro; Takano, Tatsuo; Kato, Akebumi*; Haga, Tetsuya; Yamada, Yoshikazu; Kihara, Yoshiyuki
no journal, ,
no abstracts in English
Ogawa, Shuichi*; Yamada, Takatoshi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Watanabe, Daiki*; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
no abstracts in English
Ogawa, Shuichi*; Yamada, Takatoshi*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
Ogawa, Shuichi*; Yamada, Takatoshi*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
no abstracts in English
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
no abstracts in English