Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Cs/
Cs/ Cs isotopic ratio in soil collected near Fukushima Daiichi Nuclear Power Station through mass spectrometry
Cs isotopic ratio in soil collected near Fukushima Daiichi Nuclear Power Station through mass spectrometryShimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Kim, M. S.*; Shimada, Taro; Takeda, Seiji; Yamaguchi, Tetsuji
Journal of Nuclear Science and Technology, 58(11), p.1184 - 1194, 2021/11
Times Cited Count:5 Percentile:65.59(Nuclear Science & Technology)Determining the completeness of nuclear reactor decommissioning is an important step in safely utilizing nuclear power. For example,  Cs from the Fukushima Daiichi Nuclear Power Station (FDNPS) accident can be treated as background radioactivity, so determining the origin of
Cs from the Fukushima Daiichi Nuclear Power Station (FDNPS) accident can be treated as background radioactivity, so determining the origin of  Cs is essential. To accomplish this, measuring the
Cs is essential. To accomplish this, measuring the  Cs/
Cs/ Cs isotope ratio can be useful, so this study optimized a solvent extraction method, with calix[4]arene-bis(t-octylbenzo-crown-6) [BOBCalixC6] in 1-octanol, to purify radioactive Cs, radiocesium, from a solution of major environmental soil elements and mass spectrometry interference elements. This optimized method was applied to Cs purification in soil samples (40 g), and the final solutions contained a total of 10
Cs isotope ratio can be useful, so this study optimized a solvent extraction method, with calix[4]arene-bis(t-octylbenzo-crown-6) [BOBCalixC6] in 1-octanol, to purify radioactive Cs, radiocesium, from a solution of major environmental soil elements and mass spectrometry interference elements. This optimized method was applied to Cs purification in soil samples (40 g), and the final solutions contained a total of 10 g/ml of the major soil elements and ng/ml concentrations at most of interfering elements. Soil samples collected near the FDNPS were then purified, and the
g/ml of the major soil elements and ng/ml concentrations at most of interfering elements. Soil samples collected near the FDNPS were then purified, and the  Cs/
Cs/ Cs isotope ratios were measured, using both thermal ionization mass spectrometry (TIMS) and triple quadrupole induced coupled plasma mass spectrometry (ICP-QQQ). The results of each of these measurements were compared, and we found that Cs isotope ratios obtained by TIMS were more precise, by an order of magnitude, while the ICP-QQQ results possessed good abundance sensitivities. A slightly higher
Cs isotope ratios were measured, using both thermal ionization mass spectrometry (TIMS) and triple quadrupole induced coupled plasma mass spectrometry (ICP-QQQ). The results of each of these measurements were compared, and we found that Cs isotope ratios obtained by TIMS were more precise, by an order of magnitude, while the ICP-QQQ results possessed good abundance sensitivities. A slightly higher  Cs/
Cs/ Cs ratio in the northwest area of the FDNPS was observed, while other areas exhibited similar values, all within the measurement error range, which indicated different origins of radiocesium. These results agreed with previously reported
Cs ratio in the northwest area of the FDNPS was observed, while other areas exhibited similar values, all within the measurement error range, which indicated different origins of radiocesium. These results agreed with previously reported  Cs/
Cs/ Cs activity distributions, suggesting that this ratio may be useful in identifying radiocesium origins for evaluating future nuclear reactor decommissions.
Cs activity distributions, suggesting that this ratio may be useful in identifying radiocesium origins for evaluating future nuclear reactor decommissions.
Hemmi, Ko; Walker, A.*; Yamaguchi, Tetsuji
Radiochimica Acta, 109(7), p.539 - 546, 2021/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)Plutonium(IV) sorption onto quartz in carbonate solutions was systematically investigated under anaerobic conditions to analyze the sorption behaviors of Pu(IV) with a non-electrostatic model (NEM). Pu(IV) sorption data was obtained from batch sorption experiments as a function of pH and carbonate concentration. The Pu(IV) sorption onto quartz showed similar tendencies to Th(IV), which is considered to be chemically analogous as a tetravalent actinoid. The distribution coefficient,  d, of Pu(IV) onto quartz showed inverse proportionality to the square of the total carbonate concentration under the investigated pH conditions of 8 to 11. The modeling study, however, revealed a Th(IV) sorption model, which is
d, of Pu(IV) onto quartz showed inverse proportionality to the square of the total carbonate concentration under the investigated pH conditions of 8 to 11. The modeling study, however, revealed a Th(IV) sorption model, which is  SOTh(OH)
SOTh(OH)
 and
and  SOThOH(CO
SOThOH(CO )
)
 , could not be applied to simulate the Pu(IV) sorption onto quartz. It was inferred that the electrostatic repulsion between negatively charged ligands limited the formation of
, could not be applied to simulate the Pu(IV) sorption onto quartz. It was inferred that the electrostatic repulsion between negatively charged ligands limited the formation of  SOM(OH)
SOM(OH)
 and
and  SOMOH(CO
SOMOH(CO )
)
 for Pu(IV) with smaller ionic radii than Th(IV). The Pu(IV) sorption model was developed as
for Pu(IV) with smaller ionic radii than Th(IV). The Pu(IV) sorption model was developed as  SOPu(OH)
SOPu(OH) and
and  SOPu(OH)
SOPu(OH)
 . In addition, data of Pu(IV) sorption onto muscovite was obtained in order to be compared with data for quartz.
. In addition, data of Pu(IV) sorption onto muscovite was obtained in order to be compared with data for quartz.
Yamaguchi, Tetsuji; Ohira, Saki; Hemmi, Ko; Barr, L.; Shimada, Asako; Maeda, Toshikatsu; Iida, Yoshihisa
Radiochimica Acta, 108(11), p.873 - 877, 2020/11
Times Cited Count:7 Percentile:66.68(Chemistry, Inorganic & Nuclear)Shimada, Taro; Takubo, Kazuya*; Takeda, Seiji; Yamaguchi, Tetsuji
Progress in Nuclear Science and Technology (Internet), 5, p.183 - 187, 2018/11
After fuel debris is removed from the reactor containment vessel at Fukushima Daiichi NPS (1F) and collected in waste containers in the future, the waste containers will be disposed at a deep geological repository. The uranium inventory and uranium-235 ( U) enrichment of the fuel debris are larger than those of high-level vitrified wastes which are produced from liquid waste during reprocessing of spent nuclear fuels. Therefore, there is a possibility not to be excluded that a criticality occurs in the geological media where the uranium precipitates at the far-field from the repository, after the uranium located in the repository is dissolved by groundwater. In this study, we calculated the quantity of uranium precipitated at the natural barrier, and studied dimension of uranium deposited in the natural barrier and carried out the criticality analysis.
U) enrichment of the fuel debris are larger than those of high-level vitrified wastes which are produced from liquid waste during reprocessing of spent nuclear fuels. Therefore, there is a possibility not to be excluded that a criticality occurs in the geological media where the uranium precipitates at the far-field from the repository, after the uranium located in the repository is dissolved by groundwater. In this study, we calculated the quantity of uranium precipitated at the natural barrier, and studied dimension of uranium deposited in the natural barrier and carried out the criticality analysis.
Hemmi, Ko; Yamaguchi, Tetsuji; Takeda, Seiji; Kimura, Hideo
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 24(1), p.3 - 14, 2017/06
Conditions of contaminated sources and ranges of forest decontamination that significantly reduce the air dose rate in residential areas were investigated by means of a sensitivity analysis related to the decontamination of the forest contaminated by radiocesium deriving from the accident at Fukushima Daiichi Nuclear Power Station. The contaminated sources including  Cs and
Cs and  Cs were assumed to be a layer of sedimented organic matter (the A
Cs were assumed to be a layer of sedimented organic matter (the A layer) and surface soils (the A
layer) and surface soils (the A layer). The air dose rates were calculated using the three-dimensional Monte Carlo transport code MCNP. A slope number of the forest, angles, state of contaminant distribution, radiocesium content in the forest soils, decontamination ranges, distance from the forest boundary to an evaluation point, and height at the evaluation point were adopted as the parameters. The decontamination of a litter (A
layer). The air dose rates were calculated using the three-dimensional Monte Carlo transport code MCNP. A slope number of the forest, angles, state of contaminant distribution, radiocesium content in the forest soils, decontamination ranges, distance from the forest boundary to an evaluation point, and height at the evaluation point were adopted as the parameters. The decontamination of a litter (A ) layer within the distance of 20 m from the forest boundary was revealed to be effective in reducing the air dose rate when the source distribution was homogeneous. The air dose rates were significantly reduced by the decontamination of the A
) layer within the distance of 20 m from the forest boundary was revealed to be effective in reducing the air dose rate when the source distribution was homogeneous. The air dose rates were significantly reduced by the decontamination of the A layer within a distance of 40 m from the forest boundary on condition that the radiocesium content of the A
layer within a distance of 40 m from the forest boundary on condition that the radiocesium content of the A layer was larger than that of the A
layer was larger than that of the A layer and the source distribution was non-homogeneous, such as the forest areas beyond 20 m from the forest boundary, which were more heavily contaminated than those within 20 m.
layer and the source distribution was non-homogeneous, such as the forest areas beyond 20 m from the forest boundary, which were more heavily contaminated than those within 20 m.
Iida, Yoshihisa; Nakadoi, Yasumasa; Yamaguchi, Tetsuji
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 24(1), p.53 - 64, 2017/06
The information on the contaminated water treatment in Fukushima Daiichi Nuclear Power Station announced by Tokyo Electric Power Co., Inc. (TEPCO) were summarized in terms of the management of the secondary wastes, for the purpose of accumulating technical knowledge for long-term storage of the wastes. Concerns for the long-term soundness of waste storage containers were pointed out as follows, corrosion of stainless steel containers exposed to radiation in the presence of chloride ions, corrosion of stainless steel containers under acidic conditions or in the presence of activated carbon, and radiation degradation of the high-performance container (HIC) in which slurry was stored.
 activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]Sawaguchi, Takuma; Tsukada, Manabu; Yamaguchi, Tetsuji; Mukai, Masayuki
Clay Minerals, 51(5), P. 815, 2016/12
ERRATUM; Effects of OH activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]
Iida, Yoshihisa; Yamaguchi, Tetsuji; Tanaka, Tadao; Hemmi, Ko
Journal of Nuclear Science and Technology, 53(10), p.1573 - 1584, 2016/10
Times Cited Count:10 Percentile:64.88(Nuclear Science & Technology)The sorption behavior of thorium (Th) onto granitic rock and its major constituent were investigated by batch sorption experiments. Experiments were carried out under variable pH and carbonate concentrations. Distribution coefficients decreased with increased carbonate concentrations and showed the minimal value at pH 9-10. This sorption tendency was likely due to forming the hydroxide-carbonate complexes of Th in the solutions. The order of sorbability for Th was mica  feldspar
feldspar  quartz = granite. The sorption behaviors of Th onto these minerals were analyzed by the triple-layer surface complexation model with the Visual Minteq computer program. The model calculations assuming the inner-sphere surface complexation of Th were able to explain the experimental results reasonably well. It was shown that the sorption behavior of Th onto granite can be explained primarily by the complexation with the surface sites of feldspar.
quartz = granite. The sorption behaviors of Th onto these minerals were analyzed by the triple-layer surface complexation model with the Visual Minteq computer program. The model calculations assuming the inner-sphere surface complexation of Th were able to explain the experimental results reasonably well. It was shown that the sorption behavior of Th onto granite can be explained primarily by the complexation with the surface sites of feldspar.
Iida, Yoshihisa; Barr, L.; Yamaguchi, Tetsuji; Hemmi, Ko
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 23(1), p.3 - 8, 2016/06
Thorium (Th)-229 is one of the important radionuclides for the performance assessment calculations for high-level radioactive waste repositories. The sorption behavior of Th onto montmorillonite and illite were investigated by batch sorption experiments. Experiments were carried out under variable pH and carbonate concentrations. The sorbability of montmorillonite was higher than that of illite. Distribution coefficients,  (m
(m kg
kg ), decreased with increased carbonate concentrations and showed the minimal value at around pH 10. The sorption behaviors of Th were analyzed by the non-electrostatic surface complex model with PHREEQC computer program. The model calculations were able to explain the experimental results reasonably well. The decreases of
), decreased with increased carbonate concentrations and showed the minimal value at around pH 10. The sorption behaviors of Th were analyzed by the non-electrostatic surface complex model with PHREEQC computer program. The model calculations were able to explain the experimental results reasonably well. The decreases of  was likely due to the stabilization of aqueous species by hydroxo-carbonate complexations in the solutions.
was likely due to the stabilization of aqueous species by hydroxo-carbonate complexations in the solutions.
 activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditionsSawaguchi, Takuma; Tsukada, Manabu; Yamaguchi, Tetsuji; Mukai, Masayuki
Clay Minerals, 51(2), p.267 - 278, 2016/05
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)The dependences of the dissolution rate of compacted montmorillonite on activity of OH (a
(a -) and temperature (T) were investigated. The dissolution rate of montmorillonite (
-) and temperature (T) were investigated. The dissolution rate of montmorillonite (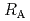 ) in compacted pure montmorillonite, which was formulized as
) in compacted pure montmorillonite, which was formulized as 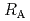 = 10
= 10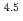 (a
(a -)
-)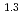 e
e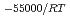 , was higher than that in the compacted sand-bentonite mixtures:
, was higher than that in the compacted sand-bentonite mixtures: 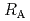 = 3500 (a
= 3500 (a -)
-)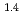 e
e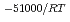 . The difference can be explained by considering the decrease in a
. The difference can be explained by considering the decrease in a - in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a
- in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a - is achieved somehow.
- is achieved somehow.
Yamaguchi, Tetsuji; Sawaguchi, Takuma; Tsukada, Manabu; Hoshino, Seiichi*; Tanaka, Tadao
Clay Minerals, 51(2), p.279 - 287, 2016/02
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)Alteration of bentonite-cement interfaces and accompanying changes in diffusivity of tritiated water was experimentally investigated using intact hardened cement specimens. The alteration by carbonate solution was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 70% of the initial value after 180-day period. Another alteration under silicate system contacting hardened cement and compacted bentonite was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 71% of the initial value after 600-day period. The changes in the diffusivity were much less than those observed for mixed specimens of granulated hardened cement and bentonite where the diffusivity decreased down to 20% of the initial value over 180 days. The results were extrapolated to 15 years under simple assumptions and showed good agreement with those observed in the cement-argillite interface at Tournemire URL. Such an explanation enhances our confidence in our assessment of alteration of cement-bentonite systems and can be a base for using our data and models in long term assessment of radioactive waste disposal.
Yamaguchi, Tetsuji; Shimada, Taro; Ishibashi, Makoto*; Akagi, Yosuke*; Kurosawa, Mitsuru*; Matsubara, Akiyoshi*; Matsuda, Yuki*; Sato, Shigeyoshi*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 22(2), p.21 - 27, 2015/12
It is predictable from previous studies that radiocesium hardly migrate into surrounding soils and groundwater from soils contaminated by the Fukushima Daiichi Nuclear Power Plant accident if they are buried and covered with indigenous soils. This study demonstrated the prediction by performing in-situ migration experiments over a year in a public park in Miho, Ibaraki prefecture and in two public parks in Misato, Saitama prefecture. Contaminated soils were buried at a depth range of 0.3 - 1.0 m or at 0.3 - 1.3 m and covered with indigenous soil layer of 0.3 m, and were sprinkled with water to accelerate the radiocesium migration. Migration of radiocesium was not observed from radiometric analyses of boring cores and soil water samples. Laboratory column and sorption experiments revealed that the radiocesium hardly leach out of the soil and even if they leach out from the contaminated soil, radiocesium is sorbed on surrounding soils and hardly migrate through the soli layer. Simulation of Cs-137 migration for 100 years by an advection-diffusion model showed that Cs-137 hardly migrate and decay out in the contaminated soil.
Hemmi, Ko; Yamaguchi, Tetsuji; Iida, Yoshihisa
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 22(1), p.3 - 10, 2015/06
Iodine and tin are important elements in performance assessment of geological disposal of radioactive wastes. Sorption experiments of iodine were carried out under varying nitrate concentration with a range of 0 to 5 mol dm at neutral pH range in order to determine the distribution coefficient of iodine was zero or non-zero value. The experimental results with estimated statistical errors showed non-zero values for tuffaceous sandstone except for NaNO
at neutral pH range in order to determine the distribution coefficient of iodine was zero or non-zero value. The experimental results with estimated statistical errors showed non-zero values for tuffaceous sandstone except for NaNO concentration 0.5 mol dm
concentration 0.5 mol dm . Non-zero values were also obtained under NaNO
. Non-zero values were also obtained under NaNO concentrations higher than 0.5 mol dm
concentrations higher than 0.5 mol dm for granodiorite. Sorption experiments of tin were carried out at high pH range in order to check whether the distribution coefficient of tin decreases significantly with pH as a result of formation of anionic hydrolysis species of tin. The distribution coefficients of tin on granodiorite decreased from 9.79
for granodiorite. Sorption experiments of tin were carried out at high pH range in order to check whether the distribution coefficient of tin decreases significantly with pH as a result of formation of anionic hydrolysis species of tin. The distribution coefficients of tin on granodiorite decreased from 9.79 10
10 m
m kg
kg at pH10.4 to 2.46
at pH10.4 to 2.46 10
10 m
m kg
kg at pH12.4. The distribution coefficient of tin on tuffaceous sandstone was about one order of magnitude higher (about 2
at pH12.4. The distribution coefficient of tin on tuffaceous sandstone was about one order of magnitude higher (about 2 10
10 m
m kg
kg ) than that of granodiorite at pH around 12.4.
) than that of granodiorite at pH around 12.4.
Yamaguchi, Tetsuji; Takeda, Seiji; Nishimura, Yuki; Iida, Yoshihisa; Tanaka, Tadao
Radiochimica Acta, 102(11), p.999 - 1008, 2014/11
Times Cited Count:1 Percentile:8.88(Chemistry, Inorganic & Nuclear)An attempt was made to select thermodynamic data with uncertainties and to evaluate the solubility of radioelements with uncertainties considering variation in groundwater chemistry. The thermodynamic data was selected by reviewing the JAEA-TDB released in 2012. Data for Nb, Pd and Pa were revised from the viewpoint of the consistency of the data selection process. Data for Se, U and Pa were revised from the viewpoint of the conservativeness. Up-to-date ternary calcium-metal(IV)-OH complexes were adopted for Zr, Th, U, Np and Pu. A Monte Carlo-based probabilistic calculation code, PA-SOL, was used for probabilistic analysis of the solubility.
Sakamaki, Keiko; Kataoka, Masaharu; Maeda, Toshikatsu; Iida, Yoshihisa; Kamoshida, Michio; Yamaguchi, Tetsuji; Tanaka, Tadao
Corrosion Engineering, Science and Technology, 49(6), p.450 - 454, 2014/09
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Corrosion experiments of a carbon steel plate embedded in bentonite mixture were conducted toverify our models assessing Eh evolution induced by corrosion of carbon steel overpack. Theexperimental results showed that the Eh decreased for the first 200 days and was subsequentlystabilised at around -450 mV; corrosion products were identified as magnetite and Fe waspresent mostly as divalent Fe within a 5 mm range from the carbon steel plate. Reactive transportmodelling was performed to assess the Eh evolution in the system using kinetic dissolution modelfor metallic iron and thermodynamic equilibrium models for other chemical reactions and closelyreproduced the experimental results. The models were verified only under the conditionsemployed in this study.
 ) onto iron-containing minerals
) onto iron-containing mineralsIida, Yoshihisa; Yamaguchi, Tetsuji; Tanaka, Tadao
Journal of Nuclear Science and Technology, 51(3), p.305 - 322, 2014/03
Times Cited Count:13 Percentile:67.4(Nuclear Science & Technology)Sorption behaviors of HSe onto iron-containing minerals, magnetite, goethite, ferrous oxide and biotite, were investigated by batch sorption experiments under reducing conditions. The sorption ratios for goethite, as representative examples, show negative dependence on pH and independence of the NaCl concentration. The results indicate that the sorption behavior of HSe
onto iron-containing minerals, magnetite, goethite, ferrous oxide and biotite, were investigated by batch sorption experiments under reducing conditions. The sorption ratios for goethite, as representative examples, show negative dependence on pH and independence of the NaCl concentration. The results indicate that the sorption behavior of HSe is inner sphere surface complexation to ferrol sites. The triple layer model was used in the analysis of the sorption behavior of HSe
is inner sphere surface complexation to ferrol sites. The triple layer model was used in the analysis of the sorption behavior of HSe with Visual Minteq computer program. The intrinsic equilibrium constant was determined by the fitting of model calculations to the experimental results to be 5.5. The value was close to the value of HS
with Visual Minteq computer program. The intrinsic equilibrium constant was determined by the fitting of model calculations to the experimental results to be 5.5. The value was close to the value of HS (5.3) which is chemically analogous to HSe
(5.3) which is chemically analogous to HSe .
.
Sawaguchi, Takuma; Kadowaki, Mitsushi*; Yamaguchi, Tetsuji; Mukai, Masayuki; Tanaka, Tadao
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 20(2), p.71 - 78, 2013/12
The dissolution rate for montmorillonite under compacted condition was studied in order to evaluate long-term alteration behavior of bentonite buffer materials by highly alkaline groundwater. The dissolution rate of compacted montmorillonite was found to be larger than that of montmorillonite in compacted sand-bentonite mixtures at 130  C, which revealed that the dissolution of montmorillonite was inhibited by decreasing the activity of hydroxide ions (
C, which revealed that the dissolution of montmorillonite was inhibited by decreasing the activity of hydroxide ions (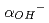 ) in the compacted mixtures including accessory minerals such as silica. In order to provide reliability for the analysis of bentonite-alteration using a formula of dissolution rate of montmorillonite, it is important to quantify the decrease of
) in the compacted mixtures including accessory minerals such as silica. In order to provide reliability for the analysis of bentonite-alteration using a formula of dissolution rate of montmorillonite, it is important to quantify the decrease of 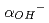 in the compacted mixtures and to formulate the dissolution rate of compacted montmorillonite.
in the compacted mixtures and to formulate the dissolution rate of compacted montmorillonite.
 C
CMaeda, Toshikatsu; Chiba, Noriaki; Tateishi, Tsuyoshi*; Yamaguchi, Tetsuji
Nihon Genshiryoku Gakkai Wabun Rombunshi, 12(2), p.158 - 164, 2013/06
no abstracts in English
Sawaguchi, Takuma; Yamaguchi, Tetsuji; Iida, Yoshihisa; Tanaka, Tadao; Kitagawa, Isamu
Clay Minerals, 48(2), p.411 - 422, 2013/05
Times Cited Count:2 Percentile:6.52(Chemistry, Physical)Diffusive transport of Cs in compacted sand-bentonite mixtures was studied by a through diffusion method. Experiments were performed under variable aqueous compositions. Effective diffusivity (
in compacted sand-bentonite mixtures was studied by a through diffusion method. Experiments were performed under variable aqueous compositions. Effective diffusivity (
 ) values of 5.2E-10
) values of 5.2E-10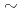 5.9E-9 m
5.9E-9 m s
s were obtained. The variation was somewhat large in the
were obtained. The variation was somewhat large in the 
 values. Apparent diffusivity (
values. Apparent diffusivity (
 ) values, on the other hand, were 2.0E-12
) values, on the other hand, were 2.0E-12  6.2E-12 m
6.2E-12 m s
s , which shows small variation. The results indicate that, in applying Fick's 1st law of diffusion, diffusive flux is proportional to the apparent concentration gradient of Cs in the sand-bentonite mixture rather than the gradient of Cs concentration in pore water. Since the apparent concentration gradient in sand-bentonite mixtures is nearly equal to the gradient of adsorbed Cs, diffusion of Cs under adsorbed state would be the main mechanism of diffusion of Cs in sand-bentonite mixtures.
, which shows small variation. The results indicate that, in applying Fick's 1st law of diffusion, diffusive flux is proportional to the apparent concentration gradient of Cs in the sand-bentonite mixture rather than the gradient of Cs concentration in pore water. Since the apparent concentration gradient in sand-bentonite mixtures is nearly equal to the gradient of adsorbed Cs, diffusion of Cs under adsorbed state would be the main mechanism of diffusion of Cs in sand-bentonite mixtures.
 concrete/clay interactions at the Tournemire URL
concrete/clay interactions at the Tournemire URLYamaguchi, Tetsuji; Kataoka, Masaharu; Sawaguchi, Takuma; Mukai, Masayuki; Hoshino, Seiichi; Tanaka, Tadao; Marsal, F.*; Pellegrini, D.*
Clay Minerals, 48(2), p.185 - 197, 2013/05
Times Cited Count:3 Percentile:9.54(Chemistry, Physical)Highly alkaline environments induced by cement based materials are likely to deteriorate the physical and/or chemical properties of the bentonite buffer materials in radioactive waste repositories. Predicting long-term alteration of concrete/clay systems requires physico-chemical models and a number of input parameters. In order to provide reliability to the long-term prediction of bentonite buffer performance under disposal conditions, it is necessary to develop and verify reactive transport codes for concrete/clay systems. In this study, a PHREEQC-based, reactive transport analysis code (MC-CEMENT ver.2) was developed and was verified by comparing results of the calculations with  observations of the mineralogical evolution at the concrete/argillite interface. The calculation reproduced the observations such as the mineralogical changes limited within one cm in thickness, formation of CaCO
observations of the mineralogical evolution at the concrete/argillite interface. The calculation reproduced the observations such as the mineralogical changes limited within one cm in thickness, formation of CaCO and CSH, dissolution of quartz, decrease of porosity in argillite and increase in concrete. These agreements indicate possibility that the models based on lab-scale (
and CSH, dissolution of quartz, decrease of porosity in argillite and increase in concrete. These agreements indicate possibility that the models based on lab-scale ( 1 y) experiments can be applied to longer time scale. The fact that the calculation did not reproduce the dissolution of clays and the formation of gypsum indicates that there is still room for improvement in our model.
1 y) experiments can be applied to longer time scale. The fact that the calculation did not reproduce the dissolution of clays and the formation of gypsum indicates that there is still room for improvement in our model.