Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Omasa, Yoshinori*; Takagi, Shigeyuki*; Toshima, Kento*; Yokoyama, Kaito*; Endo, Wataru*; Orimo, Shinichi*; Saito, Hiroyuki*; Yamada, Takeshi*; Kawakita, Yukinobu; Ikeda, Kazutaka*; et al.
Physical Review Research (Internet), 4(3), p.033215_1 - 033215_9, 2022/09
Kofu, Maiko; Watanuki, Ryuta*; Sakakibara, Toshiro*; Kawamura, Seiko; Nakajima, Kenji; Matsuura, Masato*; Ueki, Takeshi*; Akutsu, Kazuhiro*; Yamamuro, Osamu*
Scientific Reports (Internet), 11(1), p.12098_1 - 12098_8, 2021/06
Times Cited Count:5 Percentile:56.02(Multidisciplinary Sciences)Kojima, Seiji*; Novikov, V. N.*; Kofu, Maiko; Yamamuro, Osamu*
Physica Status Solidi (B), 257(11), p.200073_1 - 200073_6, 2020/11
Times Cited Count:3 Percentile:19.68(Physics, Condensed Matter)Gonzal z, M. A.*; Borodin, O.*; Kofu, Maiko; Shibata, Kaoru; Yamada, Takeshi*; Yamamuro, Osamu*; Xu, K.*; Price, D. L.*; Saboungi, M.-L.*
z, M. A.*; Borodin, O.*; Kofu, Maiko; Shibata, Kaoru; Yamada, Takeshi*; Yamamuro, Osamu*; Xu, K.*; Price, D. L.*; Saboungi, M.-L.*
Journal of Physical Chemistry Letters (Internet), 11(17), p.7279 - 7284, 2020/09
Times Cited Count:16 Percentile:77.33(Chemistry, Physical)Kofu, Maiko; Yamamuro, Osamu*
Journal of the Physical Society of Japan, 89(5), p.051002_1 - 051002_12, 2020/05
Times Cited Count:3 Percentile:31.18(Physics, Multidisciplinary)The behavior of hydrogen in metals has attracted much attention in fundamental and applied research areas for many decades. Among metals, palladium is remarkable in that it can absorb large quantities of hydrogen, and hydrogen atoms are highly mobile in the fcc Pd lattice. The dynamics of hydrogen in Pd have been investigated by means of neutron spectroscopy which is the best tool to provide insights into microscopic dynamics of hydrogen atoms. In this article, we review recent and historical neutron scattering works to facilitate the latest understanding of the hydrogen dynamics in bulk and nanometer-sized Pd hydrides.
Kojima, Seiji*; Novikov, V. N.*; Kofu, Maiko; Yamamuro, Osamu*
Journal of Non-Crystalline Solids, 518, p.18 - 23, 2019/08
Times Cited Count:6 Percentile:29.81(Materials Science, Ceramics)Nakagawa, Hiroshi; Jochi, Yasumasa*; Kitao, Akio*; Yamamuro, Osamu*; Kataoka, Mikio*
Biophysical Journal, 117(2), p.229 - 238, 2019/07
Times Cited Count:3 Percentile:13.03(Biophysics)Softness and rigidity of proteins are reflected in the structural dynamics, which are in turn affected by the environment. The characteristic low-frequency vibrational spectrum of a protein, known as boson peak, is an indication of the structural rigidity of the protein at cryogenic temperature or dehydrated conditions. In this paper, the effect of hydration, temperature, and pressure on the boson peak and volumetric properties of a globular protein are evaluated by using inelastic neutron scattering and molecular dynamics simulation. Hydration, pressurization, and cooling shift the boson peak position to higher energy and depress the peak intensity and decreases the protein and cavity volumes, although pressure hardly affects the boson peak of the fully hydrated protein. A decrease of each volume means the increase of rigidity, which is the origin of the boson peak shift. The boson peak profile can be predicted by the total cavity volume. This prediction is effective for the evaluation of the net quasielastic scattering of incoherent neutron scattering spectra when the boson peak cannot be distinguished experimentally because of a strong contribution from quasielastic scattering.
Kofu, Maiko; Faraone, A.*; Tyagi, M.*; Nagao, Michihiro*; Yamamuro, Osamu*
Physical Review E, 98(4), p.042601_1 - 042601_6, 2018/10
Times Cited Count:4 Percentile:36.54(Physics, Fluids & Plasmas)Nemoto, Fumiya*; Kofu, Maiko; Nagao, Michihiro*; Oishi, Kazuki*; Takata, Shinichi; Suzuki, Junichi*; Yamada, Takeshi*; Shibata, Kaoru; Ueki, Takeshi*; Kitazawa, Yuzo*; et al.
Journal of Chemical Physics, 149(5), p.054502_1 - 054502_11, 2018/08
Times Cited Count:19 Percentile:70.02(Chemistry, Physical)Kofu, Maiko; Hashimoto, Naoki*; Akiba, Hiroshi*; Kobayashi, Hirokazu*; Kitagawa, Hiroshi*; Iida, Kazuki*; Nakamura, Mitsutaka; Yamamuro, Osamu*
Physical Review B, 96(5), p.054304_1 - 054304_7, 2017/08
Times Cited Count:15 Percentile:58.3(Materials Science, Multidisciplinary)The vibrational states of hydrogen atoms in bulk and nanocrystalline palladium were examined in a wide energy region 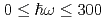 meV using neutron spectroscopy. In bulk PdH
meV using neutron spectroscopy. In bulk PdH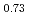 , the vibrational excitations of H atoms were roughly reproduced by the quantum harmonic oscillator (QHO) model. In PdH
, the vibrational excitations of H atoms were roughly reproduced by the quantum harmonic oscillator (QHO) model. In PdH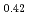 nanocrystals with a diameter of 8 nm, however, additional vibrational excitations were found at energies above 80 meV. The energies and intensities of the additional states were not explained by QHO but reasonably described as vibrations in a highly anharmonic trumpet-like potential. The additional excitations are attributed to the vibrations of H atoms at tetrahedral sites in the subsurface region stabilized by surface effects. This is an experimental work which clearly detects hydrogen vibration
nanocrystals with a diameter of 8 nm, however, additional vibrational excitations were found at energies above 80 meV. The energies and intensities of the additional states were not explained by QHO but reasonably described as vibrations in a highly anharmonic trumpet-like potential. The additional excitations are attributed to the vibrations of H atoms at tetrahedral sites in the subsurface region stabilized by surface effects. This is an experimental work which clearly detects hydrogen vibration 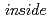 metal nanoparticles.
metal nanoparticles.
Kikuchi, Tatsuya; Nakajima, Kenji; Kawamura, Seiko; Inamura, Yasuhiro; Yamamuro, Osamu*; Kofu, Maiko*; Kawakita, Yukinobu; Suzuya, Kentaro; Nakamura, Mitsutaka; Arai, Masatoshi
Physical Review E, 87(6), p.062314_1 - 062314_8, 2013/06
Times Cited Count:18 Percentile:71.46(Physics, Fluids & Plasmas)A quasi-elastic neutron scattering (QENS) experiment is a particular technique that endeavors to define a relationship between time and space for the diffusion dynamics of atoms and molecules. However, in most cases, analyses of QENS data are model dependent. We have developed a new method for processing QENS data without a specific model, wherein all modes can be described as combinations of the relaxations based on the exponential law. By this method, we can obtain a new distribution function, 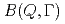 , which we call the mode distribution function (MDF), to represent the number of relaxation modes and distributions of the relaxation times in the modes. The deduction of MDF is based on the maximum entropy method. We report the first application to experimental data of liquid water. In addition to the two known modes, the existence of a new relaxation mode of water molecules with an intermediate time scale has been discovered.
, which we call the mode distribution function (MDF), to represent the number of relaxation modes and distributions of the relaxation times in the modes. The deduction of MDF is based on the maximum entropy method. We report the first application to experimental data of liquid water. In addition to the two known modes, the existence of a new relaxation mode of water molecules with an intermediate time scale has been discovered.
Shibata, Keiichi; Kawano, Toshihiko*; Nakagawa, Tsuneo; Iwamoto, Osamu; Katakura, Junichi; Fukahori, Tokio; Chiba, Satoshi; Hasegawa, Akira; Murata, Toru*; Matsunobu, Hiroyuki*; et al.
Journal of Nuclear Science and Technology, 39(11), p.1125 - 1136, 2002/11
Times Cited Count:669 Percentile:96.97(Nuclear Science & Technology)Evaluation for JENDL-3.3 has been performed by considering the accumulated feedback information and various benchmark tests of the previous library JENDL-3.2. The major problems of the JENDL-3.2 data were solved by the new library: overestimation of criticality values for thermal fission reactors was improved by the modifications of fission cross sections and fission neutron spectra for  U; incorrect energy distributions of secondary neutrons from important heavy nuclides were replaced with statistical model calculations; the inconsistency between elemental and isotopic evaluations was removed for medium-heavy nuclides. Moreover, covariance data were provided for 20 nuclides. The reliability of JENDL-3.3 was investigated by the benchmark analyses on reactor and shielding performances. The results of the analyses indicate that JENDL-3.3 predicts various reactor and shielding characteristics better than JENDL-3.2.
U; incorrect energy distributions of secondary neutrons from important heavy nuclides were replaced with statistical model calculations; the inconsistency between elemental and isotopic evaluations was removed for medium-heavy nuclides. Moreover, covariance data were provided for 20 nuclides. The reliability of JENDL-3.3 was investigated by the benchmark analyses on reactor and shielding performances. The results of the analyses indicate that JENDL-3.3 predicts various reactor and shielding characteristics better than JENDL-3.2.
Nakagawa, Hiroshi; Kataoka, Mikio; Jochi, Yasumasa*; Kitao, Akio*; Yamamuro, Osamu*
no journal, ,
no abstracts in English
Nakagawa, Hiroshi; Kataoka, Mikio; Jochi, Yasumasa*; Yamamuro, Osamu*; Nakajima, Kenji; Kawamura, Seiko
no journal, ,
Proteins are functional elements in all living organisms. Almost all vital phenomena are mediated by specific proteins. A protein is a hetero-polymer comprised of 20 types of amino acids. The sequence of amino acids of a protein is strictly determined by genetic code. When the polypeptide chain with the amino acid sequence encoded in the gene is biosynthesized, the polypeptide chain folds spontaneously into the unique tertiary structure. The tertiary structure of a protein can be determined by X-ray crystallography. Protein works in an aqueous environment at ambient temperature, indicating that protein cannot escape from thermal fluctuations. In fact, proteins are fluctuating thermally and can take some conformational substates. The magnitude of physiologically relevant input is as the same level as the thermal fluctuations. The understanding of protein dynamics is important to clarify how protein can discriminate physiologically relevant motions from random fluctuation. It is generally recognized that the internal motions of protein is essential for a protein function. Protein internal dynamics is characterized in the time scale of pico - nano second and in the space scale of the order of angstrom. Inelastic neutron scattering (INS) measurement is a powerful and unique technique for studying the protein dynamics. Here, we showed the protein dynamics studies using AGNES and AMATERAS spectrometer, which are installed at JRR-3 and J-PARC, respectively. We will discuss the hydration, temperature and pressure effect on protein dynamics as well as the difference of dynamics between folded and unfolded protein.
Kikuchi, Tatsuya; Matsumoto, Masakazu*; Yamamuro, Osamu*
no journal, ,
We are studying the formation mechanism of gas hydrates currently attracting much attention in the research field of clathrate hydrates. The largest difficulty for this study is that guest gas molecules hardly dissolve into water under ambient pressure. In order to overcome this difficulty, we prepared aqueous solutions with high solubility (2% at maximum) of guest gases by applying gas high-pressure to water. The quasi-elastic neutron scatterings (QENS) of these samples have been measured to investigate the dynamics of water molecules affected by the guest molecules. The measurements were carried out on AGNES spectrometer installed at JRR-3 (JAEA, Tokai) and maintained by ISSP, University of Tokyo. The guest molecules taken in this study were Ar, Xe, N , and CO
, and CO which have simple molecular structures. The pressure and temperature ranges were 0-100 MPa and 263-363 K, respectively. We analyzed the QENS spectra
which have simple molecular structures. The pressure and temperature ranges were 0-100 MPa and 263-363 K, respectively. We analyzed the QENS spectra 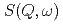 based on the jump diffusion model. The diffusion coefficient
based on the jump diffusion model. The diffusion coefficient  is smaller than
is smaller than  of pure water especially below the hydrate-formation temperature
of pure water especially below the hydrate-formation temperature 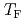 and for the guest molecules with high solubility. These experimental results were reproduced well by MD simulations. It was also found that the gas molecules get closer to each other and the diffusion of water molecules near gas molecules is suppressed, resulting in the smaller diffusion coefficient below
and for the guest molecules with high solubility. These experimental results were reproduced well by MD simulations. It was also found that the gas molecules get closer to each other and the diffusion of water molecules near gas molecules is suppressed, resulting in the smaller diffusion coefficient below 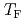 .
.
Kikuchi, Tatsuya; Nakajima, Kenji; Kawamura, Seiko; Inamura, Yasuhiro; Yamamuro, Osamu*; Kofu, Maiko*
no journal, ,
no abstracts in English
Kikuchi, Tatsuya; Nakajima, Kenji; Kawamura, Seiko; Inamura, Yasuhiro; Yamamuro, Osamu*; Kofu, Maiko*
no journal, ,
no abstracts in English
Kikuchi, Tatsuya; Nakajima, Kenji; Kawamura, Seiko; Inamura, Yasuhiro; Yamamuro, Osamu*; Kofu, Maiko*
no journal, ,
In generally so far, analysis of quasi-elastic neutron scattering spectra needs some mathematical models in its process, and hence the obtained result is a model dependent. Model-dependent analysis may lead misunderstandings caused by inappropriate initial models or may miss the unexpected phenomenon. Therefore, the alternative approach of analysis without any specific model is desired. In this context, we are trying to develop new method, which does not require any specific initial models. In this new approach, we can obtain new quantity, 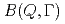 by using maximum entropy method (MEM). Our new approach was applied on liquid water. Obtained
by using maximum entropy method (MEM). Our new approach was applied on liquid water. Obtained 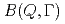 shows diffusion process in this system consist of three components: a slow motion, a fast motion and an intermediate motion between two. As it has been thought, the slow component is a translational motion and the fast component is a rotational motion. In contrast, the intermediate motion is discovered for the first time.
shows diffusion process in this system consist of three components: a slow motion, a fast motion and an intermediate motion between two. As it has been thought, the slow component is a translational motion and the fast component is a rotational motion. In contrast, the intermediate motion is discovered for the first time.
Kikuchi, Tatsuya; Nakajima, Kenji; Kawamura, Seiko; Inamura, Yasuhiro; Yamamuro, Osamu*; Kofu, Maiko*; Kawakita, Yukinobu; Suzuya, Kentaro; Nakamura, Mitsutaka; Arai, Masatoshi
no journal, ,
In generally so far, analysis of quasi-elastic neutron scattering spectra needs some mathematical models in its process, and hence the obtained result is a model dependent. In this context, we are trying to develop new model-free analysis method which we call as mode distribution analysis. In this method, we supposed that all modes can be described as combinations of the relaxations based on the exponential law. In the result of the analysis, we can obtain an intensity distribution for HWHM of Loretzian. This function can show the number of modes and distributions of the relaxation times in the modes and we call it as mode distribution function (MDF). In this new approach, we can obtain the MDF by using the maximum entropy method (MEM). We will also report a first application of our method to H O at RT. The measurement was carried out on AMATERAS spectrometer installed at J-PARC. In the result, we discover a motion of water molecule, which has not reported by earlier studies.
O at RT. The measurement was carried out on AMATERAS spectrometer installed at J-PARC. In the result, we discover a motion of water molecule, which has not reported by earlier studies.
Nakagawa, Hiroshi; Jochi, Yasumasa*; Yamamuro, Osamu*; Kataoka, Mikio
no journal, ,
Protein dynamics is closely related with protein biological function. Protein dynamical transition and boson peak is observed by neutron inelastic scattering and molecular dynamics simulation. Protein volume fluctuation is related with cavity in protein structure. The cavity volume is affected by pressure. It is important to examine the effect of pressure on protein dynamics. We examined the effect of pressure on protein dynamical transition and boson peak of Staphylococcal nuclease by neutron inelastic scattering at AGNES spectrometer at JRR-3. We found that boson peak shifted to higher energy upon pressure at 900 MP. And quasi-elastic scattering is restricted by pressure.