Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 , and NaCO
, and NaCO solutions
solutionsUehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*; Okamoto, Yoshihiro
Radiochimica Acta, 104(1), p.1 - 9, 2016/01
Times Cited Count:7 Percentile:55.03(Chemistry, Inorganic & Nuclear)The spectroelectrochemical cell was fabricated for in-situ X-ray absorption fine structure (XAFS) measurement. The XAFS spectra of U L -edge were monitored in electrolyte solutions during the electrochemical reduction. Tetravalent uranium was electrochemically prepared from hexavalent uranium and the extended X-ray absorption fine structure (EXAFS) was analyzed. The U
-edge were monitored in electrolyte solutions during the electrochemical reduction. Tetravalent uranium was electrochemically prepared from hexavalent uranium and the extended X-ray absorption fine structure (EXAFS) was analyzed. The U formed in 0.1M HNO
formed in 0.1M HNO was partially re-oxidized to UO
was partially re-oxidized to UO
 by NO
by NO
 in the solution. The UO
in the solution. The UO
 carbonates were prepared during electrolysis.
carbonates were prepared during electrolysis.

 and NpO
and NpO
 in highly concentrated chloride solutions
in highly concentrated chloride solutionsFujii, Toshiyuki*; Uehara, Akihiro*; Kitatsuji, Yoshihiro; Yamana, Hajimu*
Journal of Radioanalytical and Nuclear Chemistry, 303(1), p.1015 - 1020, 2015/01
Times Cited Count:6 Percentile:45.92(Chemistry, Analytical)The 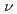
 symmetric vibrational frequency of UO
symmetric vibrational frequency of UO
 in chloride solutions was studied by Raman spectrometry and ab initio calculations. The
in chloride solutions was studied by Raman spectrometry and ab initio calculations. The 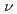
 frequency experimentally obtained decreased with the increase of concentration of Cl in solvent chlorides. This is attributable to that hydration water molecules in the equatorial plane of UO
frequency experimentally obtained decreased with the increase of concentration of Cl in solvent chlorides. This is attributable to that hydration water molecules in the equatorial plane of UO
 are substituted by Cl
are substituted by Cl ions, which was consistent with the calculation results. The theoretical part was expanded to aqua- and chloro- Np(VI) complexes. The
ions, which was consistent with the calculation results. The theoretical part was expanded to aqua- and chloro- Np(VI) complexes. The 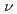
 frequencies of neptunyl species computed were acceptable compared with the reported Raman shifts.
frequencies of neptunyl species computed were acceptable compared with the reported Raman shifts.
 /UO
/UO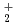 couple in Li
couple in Li MoO
MoO -Na
-Na MoO
MoO eutectic melt at 550
eutectic melt at 550 C
CNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Sato, Nobuaki*; Kofuji, Hirohide; Myochin, Munetaka; Yamana, Hajimu*
Journal of Nuclear Materials, 454(1-3), p.159 - 163, 2014/11
Times Cited Count:1 Percentile:8.88(Materials Science, Multidisciplinary)The redox equilibrium of UO /UO
/UO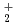 couple was measured in Li
couple was measured in Li MoO
MoO -Na
-Na MoO
MoO eutectic melt at 550
eutectic melt at 550 C by cyclic voltammetry and absorption spectrophotometry. The standard redox potential of UO
C by cyclic voltammetry and absorption spectrophotometry. The standard redox potential of UO /UO
/UO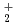 couple was approximately evaluated by cyclic voltammetry. Further, the absorption spectrum and equilibrium potential were measured, repeatedly adding UO
couple was approximately evaluated by cyclic voltammetry. Further, the absorption spectrum and equilibrium potential were measured, repeatedly adding UO source material into the melt containing UO
source material into the melt containing UO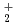 . From the correlation between the equilibrium potential of the melt and the concentration ratio [UO
. From the correlation between the equilibrium potential of the melt and the concentration ratio [UO ]/[UO
]/[UO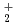 ] spectrophotometrically evaluated, the standard redox potential of UO
] spectrophotometrically evaluated, the standard redox potential of UO /UO
/UO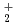 couple was determined to be -0.847
couple was determined to be -0.847 0.010 V vs. O
0.010 V vs. O /O
/O .
.

 -Ca
-Ca interaction in highly concentrated calcium chloride
interaction in highly concentrated calcium chlorideFujii, Toshiyuki*; Uehara, Akihiro*; Kitatsuji, Yoshihiro; Yamana, Hajimu*
Journal of Radioanalytical and Nuclear Chemistry, 301(1), p.293 - 296, 2014/07
Times Cited Count:6 Percentile:42.97(Chemistry, Analytical)Coordination circumstance of neptunyl ion in concentrated CaCl solutions was analyzed by Raman spectrometry. Besides the symmetric stretch (
solutions was analyzed by Raman spectrometry. Besides the symmetric stretch (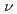
 ) mode of NpO
) mode of NpO
 and NpO
and NpO
 , the asymmetric stretch (
, the asymmetric stretch (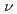
 ) mode of NpO
) mode of NpO
 was found. The Raman intensity of the
was found. The Raman intensity of the 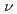
 mode increased with the concentration of CaCl
mode increased with the concentration of CaCl in the system. This would be attributable to the cation-cation interaction between Np(V) and Ca(II).
in the system. This would be attributable to the cation-cation interaction between Np(V) and Ca(II).

 in molten LiCl-RbCl and LiCl-KCl eutectics by using tungsten
in molten LiCl-RbCl and LiCl-KCl eutectics by using tungstenNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*
Journal of Nuclear Materials, 439(1-3), p.1 - 6, 2013/08
Times Cited Count:8 Percentile:49.28(Materials Science, Multidisciplinary)The reduction of uranium from UO
 to UO
to UO
 or U
or U in molten LiCl-RbCl and LiCl-KCl eutectics was examined by using tungsten and chlorine gas. Spectrophotometric technique was adopted to determine the concentration of uranium species. When tungsten was immersed into the LiCl-RbCl eutectic melt at 400
in molten LiCl-RbCl and LiCl-KCl eutectics was examined by using tungsten and chlorine gas. Spectrophotometric technique was adopted to determine the concentration of uranium species. When tungsten was immersed into the LiCl-RbCl eutectic melt at 400 C without supplying chlorine gas, 36% of the total weight of the hexavalent of UO
C without supplying chlorine gas, 36% of the total weight of the hexavalent of UO
 was reduced to the pentavalent of UO
was reduced to the pentavalent of UO
 . Under purging chlorine gas into the melt, 96% of UO
. Under purging chlorine gas into the melt, 96% of UO
 was reduced to the tetravalent of U
was reduced to the tetravalent of U . Tungsten oxy-chloride of WOCl
. Tungsten oxy-chloride of WOCl was produced via the reductions of UO
was produced via the reductions of UO
 , which was volatized from the melt and adsorbed on the upper part of experimental cell. On the other hand, 84% of UO
, which was volatized from the melt and adsorbed on the upper part of experimental cell. On the other hand, 84% of UO
 in the LiCl-KCl eutectic melt at 500
in the LiCl-KCl eutectic melt at 500 C was reduced to U
C was reduced to U by using tungsten and chlorine gas.
by using tungsten and chlorine gas.
Fujii, Toshiyuki*; Egusa, Soichiro*; Uehara, Akihiro*; Yamana, Hajimu*; Morita, Yasuji
Journal of Radioanalytical and Nuclear Chemistry, 295(3), p.2059 - 2062, 2013/03
Times Cited Count:3 Percentile:18.63(Chemistry, Analytical)Quantitative analysis of Nd, U, or Pd in 3 mol dm (M) HNO
(M) HNO was performed by reflection absorption spectrophotometry at ultraviolet-visible-near-infrared (UV/Vis/NIR) region. A sample chamber with optics for reflection measurement was designed and attached to a UV/Vis/NIR spectrophotometer by optical fibers. The reflection absorbance showed linear relations with concentrations of Nd, U, and Pd at the absorbance region less than 0.1. The quantitative analysis was found to be possible for 3 M HNO
was performed by reflection absorption spectrophotometry at ultraviolet-visible-near-infrared (UV/Vis/NIR) region. A sample chamber with optics for reflection measurement was designed and attached to a UV/Vis/NIR spectrophotometer by optical fibers. The reflection absorbance showed linear relations with concentrations of Nd, U, and Pd at the absorbance region less than 0.1. The quantitative analysis was found to be possible for 3 M HNO solutions containing [Nd] ca. 0.2 M, [U] ca. 0.04 M, or [Pd] ca. 0.01 M at maximum.
solutions containing [Nd] ca. 0.2 M, [U] ca. 0.04 M, or [Pd] ca. 0.01 M at maximum.
 MoO
MoO
Nagai, Takayuki; Sato, Nobuaki*; Kitawaki, Shinichi; Uehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*; Myochin, Munetaka
Journal of Nuclear Materials, 433(1-3), p.397 - 403, 2013/02
Times Cited Count:10 Percentile:61.16(Materials Science, Multidisciplinary)In order to examine easily synthetic conditions of uranyl molybdate, UO MoO
MoO , used for the reprocessing process study of spent nuclear oxide fuels in alkaline molybdate melts, the uranium molybdate compounds were produced from U
, used for the reprocessing process study of spent nuclear oxide fuels in alkaline molybdate melts, the uranium molybdate compounds were produced from U O
O powder and anhydrous MoO
powder and anhydrous MoO reagent. The results of having investigated them in solid state by using X-ray diffractometry and Raman spectrometry, it was confirmed that UO
reagent. The results of having investigated them in solid state by using X-ray diffractometry and Raman spectrometry, it was confirmed that UO MoO
MoO could be synthesized by heating mixed powder of U
could be synthesized by heating mixed powder of U O
O and MoO
and MoO with stoichiometric mole ratio at 770
with stoichiometric mole ratio at 770 C for 4 h under air atmosphere. Moreover, adding this UO
C for 4 h under air atmosphere. Moreover, adding this UO MoO
MoO into Li
into Li MoO
MoO -Na
-Na MoO
MoO eutectic melt, most of the dissolved uranium species in the melt were observed as hexa-valent uranyl ions by absorption spectrophotometry.
eutectic melt, most of the dissolved uranium species in the melt were observed as hexa-valent uranyl ions by absorption spectrophotometry.
Osaka, Masahiko; Donomae, Takako; Ichikawa, Shoichi; Sasaki, Shinji; Ishimi, Akihiro; Inoue, Toshihiko; Sekio, Yoshihiro; Miwa, Shuhei; Onishi, Takashi; Asaka, Takeo; et al.
Proceedings of 1st Asian Nuclear Fuel Conference (ANFC), 2 Pages, 2012/03
Support system for training and education of future expert in hot laboratories of Oarai-JAEA, named FEETS, is presented. The system has been established based on research results on both characterization of Oarai hot laboratory and user-needs. Various programs under FEETS are also introduced.
Osaka, Masahiko; Konashi, Kenji*; Hayashi, Hirokazu; Li, D.*; Homma, Yoshiya*; Yamamura, Tomoo*; Sato, Isamu; Miwa, Shuhei; Sekimoto, Shun*; Kubota, Takumi*; et al.
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Summer schools for future experts have successfully been completed under Japan Actinide Network (J-ACTINET) for the purpose of development of human resources who are expected to be engaged in every areas of actinide-research/engineering. The first summer school was held in Ibaraki-area in August 2009, followed by the second one in Kansai-area in August 2010. Two summer schools have focused on actual experiences of actinides in actinide-research fields for university students and young researchers/engineers as an introductory course of actinide-researches. Several quasi actinide-handling experiences at the actinide-research fields have attracted attentions of participants at the first school in Ibaraki-area. The actual experiments using actinides-containing solutions have been carried out at the second school in Kansai-area. Future summer schools will be held every year for the sustainable human resource development in various actinide-research fields.
Minato, Kazuo; Konashi, Kenji*; Yamana, Hajimu*; Yamanaka, Shinsuke*; Nagasaki, Shinya*; Ikeda, Yasuhisa*; Sato, Seichi*; Arita, Yuji*; Idemitsu, Kazuya*; Koyama, Tadafumi*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Actinide science research is indispensable to maintain sustainable development of innovative nuclear technology. For actinide science research, special facilities with containment and radiation shields are needed to handle actinide materials. The number of facilities for actinide science research has been decreased, especially in universities, due to the high maintenance cost. J-ACTINET was established in 2008 to promote and facilitate actinide science research and to foster many of young scientists and engineers in actinide science. The research program was carried out, through which young researchers were expected to learn how to make experiments with advanced experimental tools and to broaden their horizons. The summer schools and computational science school were held to provide students and young researchers with the opportunities to come into contact with actinide science research. The overseas dispatch program was also carried out.
Fujii, Toshiyuki*; Egusa, Soichiro*; Uehara, Akihiro*; Kirishima, Akira*; Yamagishi, Isao; Morita, Yasuji; Yamana, Hajimu*
Journal of Radioanalytical and Nuclear Chemistry, 290(2), p.475 - 478, 2011/11
Times Cited Count:19 Percentile:80.18(Chemistry, Analytical)Palladium complexation in concentrated nitric acid solutions was studied by UV/Vis absorption spectrophotometry. The ionic strength of the solutions was fixed to 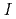 = 1, 3, or 5 M by mixing of HNO
= 1, 3, or 5 M by mixing of HNO and HClO
and HClO . The major palladium species were found to be Pd
. The major palladium species were found to be Pd , PdNO
, PdNO
 , and Pd(NO
, and Pd(NO )
) . The formation constant of PdNO
. The formation constant of PdNO
 was determined to be
was determined to be 
 = 1.32 (
= 1.32 (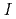 = 1 M), 1.49 (
= 1 M), 1.49 (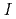 = 3 M), or 1.47 (
= 3 M), or 1.47 (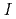 = 5 M), while that of Pd(NO
= 5 M), while that of Pd(NO )
) to be
to be 
 = 0.45 (
= 0.45 (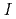 = 3 M) or 0.14 (
= 3 M) or 0.14 (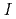 = 5 M).
= 5 M).
 C
CNagai, Takayuki; Uehara, Akihiro*; Fukushima, Mineo; Myochin, Munetaka; Fujii, Toshiyuki*; Sato, Nobuaki*; Yamana, Hajimu*
Proceedings in Radiochemistry, 1(1), p.151 - 155, 2011/09
Absorption spectra of dissolved uranium species in molten Li MoO
MoO -Na
-Na MoO
MoO eutectic at 550
eutectic at 550  C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
 in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
 decreased and UO
decreased and UO
 was thought to be oxidized to UO
was thought to be oxidized to UO
 . When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO
. When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.
was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.
 solutions
solutionsUehara, Akihiro*; Fujii, Toshiyuki*; Matsuura, Haruaki*; Sato, Nobuaki*; Nagai, Takayuki; Minato, Kazuo; Yamana, Hajimu*; Okamoto, Yoshihiro
Proceedings in Radiochemistry, 1(1), p.161 - 165, 2011/09
Nagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Sato, Nobuaki*; Yamana, Hajimu*
Journal of Nuclear Materials, 414(2), p.226 - 231, 2011/07
Times Cited Count:11 Percentile:64.09(Materials Science, Multidisciplinary) )
) and CaCl
and CaCl
Fujii, Toshiyuki*; Okude, Genki*; Uehara, Akihiro*; Sekimoto, Shun*; Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo; Yamana, Hajimu*
Journal of Radioanalytical and Nuclear Chemistry, 288(1), p.181 - 187, 2011/04
Times Cited Count:6 Percentile:44.28(Chemistry, Analytical)Distribution behavior of Ce(III), Am(III), and Cm(III) between tri-n-butyl phosphate solution and molten calcium nitrate hydrate Ca(NO )
)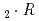 H
H O or molten calcium chloride hydrate CaCl
O or molten calcium chloride hydrate CaCl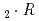 H
H O was studied radiochemically. In Ca(NO
O was studied radiochemically. In Ca(NO )
)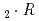 H
H O systems, maximum separation factors of Ce and Cm to Am were observed to be 12 (Ce/Am) and 1.7 (Cm/Am). The distribution ratios of these elements increased with the decrease of water activity in the hydrates, and the extractabilities at the water deficient region was less sensitive compared to those at the water abundant region. This trend was similar to the coordination circumstance change observed in electronic absorption spectra of Nd(III) in the hydrates.
O systems, maximum separation factors of Ce and Cm to Am were observed to be 12 (Ce/Am) and 1.7 (Cm/Am). The distribution ratios of these elements increased with the decrease of water activity in the hydrates, and the extractabilities at the water deficient region was less sensitive compared to those at the water abundant region. This trend was similar to the coordination circumstance change observed in electronic absorption spectra of Nd(III) in the hydrates.
Egusa, Soichiro*; Fujii, Toshiyuki*; Uehara, Akihiro*; Yamana, Hajimu*; Yamagishi, Isao; Morita, Yasuji
Kyoto Daigaku Genshiro Jikkenjo Dai-45-Kai Gakujutsu Koenkai Hobunshu, p.123 - 125, 2011/01
Speciation of palladium in nitric acid solutions was studied through spectrophotometric methods to obtain basic data for development of Pd separation process. Stability constants of nitrate complexes of Pd were determined by analysis of absorption spectra of nitric acid solutions of various concentrations with the constant ionic strength.
Okude, Genki*; Fujii, Toshiyuki*; Uehara, Akihiro*; Sekimoto, Shun*; Minato, Kazuo; Yamana, Hajimu*
IOP Conference Series; Materials Science and Engineering, 9, p.012067_1 - 012067_7, 2010/05
Times Cited Count:2 Percentile:70.97(Chemistry, Inorganic & Nuclear) /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic
/U couples in molten LiCl-RbCl eutecticNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Shirai, Osamu*; Sato, Nobuaki*; Yamana, Hajimu*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.614 - 616, 2009/08
Times Cited Count:14 Percentile:31.08(Electrochemistry)Formal redox potentials of the U /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but positive comparing to those in the NaCl-CsCl eutectic. This relation would be correlated with the averaged alkali cation radius.
/U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but positive comparing to those in the NaCl-CsCl eutectic. This relation would be correlated with the averaged alkali cation radius.
 /Pu
/Pu and PuO
and PuO
 /Pu
/Pu couples in molten NaCl-CsCl eutectic as measured by absorption spectrophotometry
couples in molten NaCl-CsCl eutectic as measured by absorption spectrophotometryNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Shirai, Osamu*; Myochin, Munetaka; Yamana, Hajimu*
Radiochimica Acta, 97(4-5), p.209 - 212, 2009/05
Times Cited Count:3 Percentile:24.52(Chemistry, Inorganic & Nuclear)Redox behavior of uranium and plutonium in molten salts is the essential information for establishing an effective reprocessing process with spent MOX fuel. In an oxide electro-winning reprocessing process, the spent nuclear fuel is dissolved into the molten NaCl-CsCl eutectic, and the dissolved U(VI) and Pu(VI) species are reduced to be dioxides by electrolysis. In the present study, formal redox potentials of the Pu /Pu
/Pu and PuO
and PuO
 /Pu
/Pu couples in the molten NaCl-CsCl eutectic at 923 K were determined. The fractions of Pu
couples in the molten NaCl-CsCl eutectic at 923 K were determined. The fractions of Pu , Pu
, Pu , and PuO
, and PuO
 were determined by UV/Vis/NIR absorption spectrophotometry. PuO
were determined by UV/Vis/NIR absorption spectrophotometry. PuO was dissolved into the molten NaCl-CsCl eutectic by Cl
was dissolved into the molten NaCl-CsCl eutectic by Cl
 gas purging. Valences of plutonium (Pu
gas purging. Valences of plutonium (Pu , Pu
, Pu , Pu
, Pu , and PuO
, and PuO
 ) were controlled by changing the flow rates of Cl
) were controlled by changing the flow rates of Cl , O
, O , and Ar gases.
, and Ar gases.
 /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic
/U couples in molten LiCl-RbCl eutecticNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Shirai, Osamu*; Sato, Nobuaki*; Yamana, Hajimu*
Proceedings of 2008 Joint Symposium on Molten Salts (USB Flash Drive), p.927 - 932, 2008/10
Formal redox potentials of the U /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but approximately similar to those in the NaCl-CsCl eutectic. These redox potentials in molten alkali chlorides would be correlated with the averaged alkali cationic radius of the melt.
/U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but approximately similar to those in the NaCl-CsCl eutectic. These redox potentials in molten alkali chlorides would be correlated with the averaged alkali cationic radius of the melt.