Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
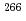 Bh in the
Bh in the  Cm(
Cm(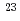 Na,5
Na,5 )
)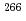 Bh reaction and its decay properties
Bh reaction and its decay propertiesHaba, Hiromitsu*; Fan, F.*; Kaji, Daiya*; Kasamatsu, Yoshitaka*; Kikunaga, Hidetoshi*; Komori, Yukiko*; Kondo, Narumi*; Kudo, Hisaaki*; Morimoto, Koji*; Morita, Kosuke*; et al.
Physical Review C, 102(2), p.024625_1 - 024625_12, 2020/08
Times Cited Count:7 Percentile:55.65(Physics, Nuclear)Oe, Kazuhiro*; Attallah, M. F.*; Asai, Masato; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke*; Kasamatsu, Yoshitaka*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1317 - 1320, 2015/02
Times Cited Count:10 Percentile:60.68(Chemistry, Analytical)A new technique for continuous dissolution of nuclear reaction products transported by a gas-jet system was developed for superheavy element (SHE) chemistry. In this technique, a hydrophobic membrane is utilized to separate an aqueous phase from the gas phase. With this technique, the dissolution efficiencies of short-lived radionuclides of 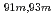 Mo and
Mo and 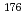 W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
Komori, Yukiko*; Yokokita, Takuya*; Kasamatsu, Yoshitaka*; Haba, Hiromitsu*; Toyoshima, Atsushi; Toyomura, Keigo*; Nakamura, Kohei*; Kanaya, Jumpei*; Huang, M.*; Kudo, Yuki*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1385 - 1388, 2015/02
Times Cited Count:2 Percentile:16.17(Chemistry, Analytical)We studied solid-liquid extraction behavior of carrier-free Mo and W radiotracers onto an Aliquat 336-loaded resin from HCl solutions toward extraction chromatography experiments of element 106, seaborgium(Sg). Distribution coefficients (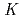
 ) of Mo and W on the resin were determined as a function of HCl concentration by a batch method. On-line extraction chromatography of Mo and W was also carried out in 2-8 M HCl solutions with an automated rapid chemistry apparatus. The order of extraction probability from 2 to 8 M HCl was Mo
) of Mo and W on the resin were determined as a function of HCl concentration by a batch method. On-line extraction chromatography of Mo and W was also carried out in 2-8 M HCl solutions with an automated rapid chemistry apparatus. The order of extraction probability from 2 to 8 M HCl was Mo W, which reflected the order of the
W, which reflected the order of the 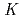
 values in the batch experiment.
values in the batch experiment.
Toda, Kosuke*; Ueno, Shingo*; Takahashi, Naruto*; Kasamatsu, Yoshitaka*; Yokokita, Takuya*; Oe, Kazuhiro; Yokoyama, Akihiko*
no journal, ,
In order to study the effect of the degree of deformation on the fusion reaction, we have researched for the fusion reaction of  Lu +
Lu + 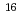 O and
O and 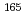 Ho +
Ho + 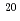 Ne. In the systems, the projectile nucleus
Ne. In the systems, the projectile nucleus 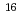 O is spherical, while
O is spherical, while 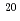 Ne is deformed. Thus they are fusion reactions of deformed-spherical nuclei and deformed-deformed nuclei, respectively. Besides, latter system has the same compound nucleus,
Ne is deformed. Thus they are fusion reactions of deformed-spherical nuclei and deformed-deformed nuclei, respectively. Besides, latter system has the same compound nucleus, 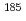 Ir, as the
Ir, as the 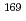 Tm +
Tm + 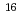 O reaction in previous work. The evaporation residue cross sections were measured by using a stack technique and a gas-jet technique for the projectile energies of 50 to 155 MeV. The cross sections were compared with the theoretical values calculated by HIVAP code. The measured excitation functions were found to agree fairly well with theoretical values. The results are consistent with the idea that the degree of deformation makes an effect on the rising edge of excitation functions near the Coulomb barrier.
O reaction in previous work. The evaporation residue cross sections were measured by using a stack technique and a gas-jet technique for the projectile energies of 50 to 155 MeV. The cross sections were compared with the theoretical values calculated by HIVAP code. The measured excitation functions were found to agree fairly well with theoretical values. The results are consistent with the idea that the degree of deformation makes an effect on the rising edge of excitation functions near the Coulomb barrier.
Yokokita, Takuya*; Oe, Kazuhiro; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Nakamura, Kohei*; Kasamatsu, Yoshitaka*; Takahashi, Naruto*; Yoshimura, Takashi*; Takamiya, Koichi*; et al.
no journal, ,
We carried out solvent extraction of Mo(VI), Mo(V), W(VI) and W(V) in 0.01-0.36 M Aliquat 336 / 0.1-11 M HCl system as model experiments for element 106, seaborgium (Sg). The HCl solutions of Mo(VI), Mo(V), W(VI) and W(V) were prepared using macro amounts of Mo and W. Extraction behaviors of mononuclear Mo and W were also investigated using carrier-free radiotracers  Mo and
Mo and  W, which were produced as
W, which were produced as  U(n, f) and
U(n, f) and  Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
Toyoshima, Atsushi; Miyashita, Sunao*; Asai, Masato; Sato, Tetsuya; Kaneya, Yusuke; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Nagame, Yuichiro; Sch del, M.; Lerum, H. V.*; et al.
del, M.; Lerum, H. V.*; et al.
no journal, ,
To carry out a continuous reduction experiment of Sg with the low production rates and the short half-life, we are developing a new chemistry assembly consisting of a membrane degasser (MDG), a flow electrolytic column (FEC), the continuous liquid-liquid extraction apparatus, and the liquid scintillation counting system (SISAK). Recently, we have begun preparatory studies with Mo and W isotopes. Aqueous solution dissolving Mo and W was successfully separated from a carrier gas. Redox couples of Mo(VI)/Mo(V) and W(VI)/W(V) in HCl have been characterized for their macro amounts. Extraction behavior of Mo(VI) and W(VI) between toluene containing hinokitiol (HT) and HCl was successfully observed by a batch method. On-line extractions of short-lived Mo and W were also carried out using SISAK and MDG. In the symposium, our present status of the preparation with Mo and W will be presented.
 solution for understanding chemical species of Db
solution for understanding chemical species of DbAdachi, Sadia*; Sueki, Keisuke*; Toyoshima, Atsushi*; Tsukada, Kazuaki; Haba, Hiromitsu*; Komori, Yukiko*; Yokokita, Takuya*; Mori, Taiki*
no journal, ,
Anion exchange behavior of group-5 elements, Nb, Ta, and their pseudo homologue Pa in HF and HF/HNO solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO
solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO
aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.
concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.
Toyoshima, Atsushi; Oe, Kazuhiro*; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Kaneko, Masashi*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Due to short half-lives less than 10 s and extremely low production rates, transactinide elements heavier than seaborgium (Sg) are produced on an atom per hour scale. Therefore, a continuous rapid chemistry assembly is required to study aqueous-phase chemistry of these heaviest elements. In the present study, we started developments of a continuous chemistry assembly. Our first attempt was made in on-line experiments with Mo and W, lighter homologs of Sg, to optimize a chemistry assembly consisting of a newly developed membrane degasser as an interface between gas-jet and aqueous phase, a flow electrolytic column apparatus utilized to control oxidation states of Mo and W ions, and the continuous liquid-liquid extraction apparatus of SISAK for separation. In the conference, present status of the developments will be presented.
 solution
solutionKato, Mizuho*; Adachi, Sadia*; Toyoshima, Atsushi*; Tsukada, Kazuaki; Asai, Masato; Haba, Hiromitsu*; Yokokita, Takuya*; Komori, Yukiko*; Shigekawa, Yudai*; Sueki, Keisuke*
no journal, ,
A few studies have been so far performed on aqueous chemistry of Db, the group-5 superheavy element, by anion-exchange experiments in HF and HF/HNO mixture solutions. Although the distribution coefficient (Kd) of Db was shown to trend to Ta
mixture solutions. Although the distribution coefficient (Kd) of Db was shown to trend to Ta  Nb
Nb  Db
Db  Pa in the group-5 homologs and pseudo-homolog elements, its chemical species has not been clarified. In this work, for identification of fluoride species of Db, we performed anion-exchange experiments of Nb, Ta, and Db in HF/1.0 M HNO
Pa in the group-5 homologs and pseudo-homolog elements, its chemical species has not been clarified. In this work, for identification of fluoride species of Db, we performed anion-exchange experiments of Nb, Ta, and Db in HF/1.0 M HNO mixture solutions by using an automated rapid chemistry apparatus, ARCA.
mixture solutions by using an automated rapid chemistry apparatus, ARCA.
Toyoshima, Atsushi; Miyashita, Sunao*; Oe, Kazuhiro*; Kitayama, Yuta*; Lerum, H. V.*; Goto, Naoya*; Kaneya, Yusuke; Komori, Yukiko*; Mitsukai, Akina*; Vascon, A.; et al.
no journal, ,
no abstracts in English
Toyoshima, Atsushi; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Towards electrolytic reduction of Sg, batch-wise electrolytic reduction of carrier-free 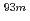 Mo and
Mo and 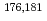 W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior.
W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior. 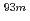 Mo and
Mo and 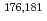 W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
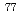 Br using the CCONE-based calculation system
Br using the CCONE-based calculation systemSakai, Seiya*; Otsu, Hideaki*; Iwamoto, Osamu; Iwamoto, Nobuyuki; Nakayama, Shinsuke; Fukahori, Tokio; Kikunaga, Hidetoshi*; Yokokita, Takuya*
no journal, ,
no abstracts in English
Yokokita, Takuya*; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Shiohara, Naoya*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; Oe, Kazuhiro; Shinohara, Atsushi*
no journal, ,
We are planning to study a redox behavior of element 106, seaborgium (Sg) based on the comparison of the behaviors with those of molybdenum (Mo) and tungsten (W). In this work, we studied solvent extraction behaviors of Mo(VI), Mo(V), W(VI), and W(V) with Aliquat 336 from hydrochloric acid (HCl). Mo(V) is extracted with higher distribution ratios than those of Mo(VI) in 4 to 11 M HCl. Distribution ratios of Mo(V) obtained by reducing Mo(VI) electrochemically are in good agreement with those of [MoCl ].
].