Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
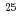 F from
F from  O
O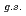 ?
?Tang, T. L.*; Uesaka, Tomohiro*; Kawase, Shoichiro; Beaumel, D.*; Dozono, Masanori*; Fujii, Toshihiko*; Fukuda, Naoki*; Fukunaga, Taku*; Galindo-Uribarri, A.*; Hwang, S. H.*; et al.
Physical Review Letters, 124(21), p.212502_1 - 212502_6, 2020/05
Times Cited Count:14 Percentile:74.18(Physics, Multidisciplinary)The structure of a neutron-rich 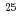 F nucleus is investigated by a quasifree (
F nucleus is investigated by a quasifree (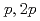 ) knockout reaction. The sum of spectroscopic factors of
) knockout reaction. The sum of spectroscopic factors of 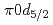 orbital is found to be 1.0
orbital is found to be 1.0  0.3. The result shows that the
0.3. The result shows that the  O core of
O core of 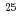 F nucleus significantly differs from a free
F nucleus significantly differs from a free  O nucleus, and the core consists of
O nucleus, and the core consists of  35%
35%  O
O , and
, and  65% excited
65% excited  O. The result shows that the
O. The result shows that the  O core of
O core of  F nucleus significantly differs from a free
F nucleus significantly differs from a free  O nucleus. The result may infer that the addition of the
O nucleus. The result may infer that the addition of the 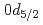 proton considerably changes the neutron structure in
proton considerably changes the neutron structure in 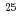 F from that in
F from that in  O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
Tanaka, Taiki*; Narikiyo, Yoshihiro*; Morita, Kosuke*; Fujita, Kunihiro*; Kaji, Daiya*; Morimoto, Koji*; Yamaki, Sayaka*; Wakabayashi, Yasuo*; Tanaka, Kengo*; Takeyama, Mirei*; et al.
Journal of the Physical Society of Japan, 87(1), p.014201_1 - 014201_9, 2018/01
Times Cited Count:18 Percentile:74.47(Physics, Multidisciplinary)Excitation functions of quasielastic scattering cross sections for the  Ca +
Ca +  Pb,
Pb,  Ti +
Ti +  Pb, and
Pb, and  Ca +
Ca +  Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the
Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the  Ca +
Ca +  Pb and
Pb and  Ti +
Ti +  Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the
Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the  Ca +
Ca +  Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
Ishibashi, Masayuki; Ando, Tomomi*; Sasao, Eiji; Yuguchi, Takashi; Nishimoto, Shoji*; Yoshida, Hidekazu*
Oyo Chishitsu, 55(4), p.156 - 165, 2014/10
Understanding of long-term history of water-conducting features such as flow-path fractures is key issue to evaluate deep geological environment for geological disposal of high-level radioactive waste (HLW). Thus, we conducted study on the geological features and the long-term behavior of flow-path fractures based on the data obtained at -300m levels in the Mizunami Underground research laboratory (MIU), central Japan. Total 1670 fractures were mapped in underground gallery at the -300m levels. Flow-path fractures occupy about 11% of all fractures. The flow-path fractures are divided into grout filling fractures and low inflow-rate fractures. All of the grout filling fractures is filled with calcite as fracture filling minerals without conspicuous host rock alteration around fractures. The low inflow-rate fractures possessed similar geological character with the sealed fractures which are not acted as flow-path. The geological character of fracture filling and host tock alteration around fractures indicates the history of the formation at the time of intrusion and emplacement of host granite (Stage I), then filling at hydrothermal event (Stage II), and finally opening and elongation during exhumation stage (Stage III). In conclusion, the present flow-path fractures were formed by opening and/or elongation of pre-existed fractures, which were filled at the hydrothermal event, at the time of exhumation.