Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
 solutions; Model experiments for the chemical study of Db
solutions; Model experiments for the chemical study of DbKasamatsu, Yoshitaka; Toyoshima, Atsushi; Haba, Hiromitsu*; Tome, Hayato*; Tsukada, Kazuaki; Akiyama, Kazuhiko*; Yoshimura, Takashi*; Nagame, Yuichiro
Journal of Radioanalytical and Nuclear Chemistry, 279(2), p.371 - 376, 2009/02
Times Cited Count:13 Percentile:63.16(Chemistry, Analytical)Anion-exchange behavior of the group-5 elements Nb and Ta, and their pseudo homologue Pa in HF and HF/HNO solutions was investigated by a batch method to find suitable conditions for the anion-exchange experiment of element 105 (Dubnium, Db). We determined distribution coefficients of those elements on the anion-exchange resin as a function of the F
solutions was investigated by a batch method to find suitable conditions for the anion-exchange experiment of element 105 (Dubnium, Db). We determined distribution coefficients of those elements on the anion-exchange resin as a function of the F and NO
and NO
 concentrations. Clearly different anion-exchange behavior was observed among these elements. Based on the results, we discuss the fluoro-complex formation of each element and suggest experimental conditions for the study of fluoride complexation of Db.
concentrations. Clearly different anion-exchange behavior was observed among these elements. Based on the results, we discuss the fluoro-complex formation of each element and suggest experimental conditions for the study of fluoride complexation of Db.
 Ni via the (
Ni via the (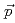 ,
, 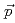 ') reaction at 0
') reaction at 0
Ishikawa, Takatsugu*; Akimune, Hidetoshi*; Daito, Izuru*; Fujimura, Hisako*; Fujita, Yoshitaka*; Fujiwara, Mamoru; Hatanaka, Kichiji*; Hosono, K.*; Ihara, F.*; Ito, M.*; et al.
Nuclear Physics A, 187(1-2), p.58c - 63c, 2001/04
no abstracts in English
Yokokita, Takuya*; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Shiohara, Naoya*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; Oe, Kazuhiro; Shinohara, Atsushi*
no journal, ,
We are planning to study a redox behavior of element 106, seaborgium (Sg) based on the comparison of the behaviors with those of molybdenum (Mo) and tungsten (W). In this work, we studied solvent extraction behaviors of Mo(VI), Mo(V), W(VI), and W(V) with Aliquat 336 from hydrochloric acid (HCl). Mo(V) is extracted with higher distribution ratios than those of Mo(VI) in 4 to 11 M HCl. Distribution ratios of Mo(V) obtained by reducing Mo(VI) electrochemically are in good agreement with those of [MoCl ].
].
Yokokita, Takuya*; Oe, Kazuhiro; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Nakamura, Kohei*; Kasamatsu, Yoshitaka*; Takahashi, Naruto*; Yoshimura, Takashi*; Takamiya, Koichi*; et al.
no journal, ,
We carried out solvent extraction of Mo(VI), Mo(V), W(VI) and W(V) in 0.01-0.36 M Aliquat 336 / 0.1-11 M HCl system as model experiments for element 106, seaborgium (Sg). The HCl solutions of Mo(VI), Mo(V), W(VI) and W(V) were prepared using macro amounts of Mo and W. Extraction behaviors of mononuclear Mo and W were also investigated using carrier-free radiotracers  Mo and
Mo and  W, which were produced as
W, which were produced as  U(n, f) and
U(n, f) and  Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
Nishino, Hiroyuki; Kurisaka, Kenichi; Yoshimura, Kazuo; Nishimura, Masahiro; Fukano, Yoshitaka
no journal, ,
no abstracts in English
Nishimura, Masahiro; Yoshimura, Kazuo; Taninaka, Hiroshi; Yamada, Fumiaki; Mori, Takero; Nishino, Hiroyuki; Fukano, Yoshitaka
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Yang, Y.*; Fujisaki, Kenta*; Kawaguchi, Yuko*; Kobayashi, Kensei*; Hashimoto, Hirofumi*; Kawai, Hideyuki*; Mita, Hajime*; Narumi, Issei; Okudaira, Kyoko*; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Yang, Y.*; Fujisaki, Kenta*; Kawaguchi, Yuko*; Hashimoto, Hirofumi*; Yamashita, Masamichi*; Yano, Hajime*; Okudaira, Kyoko*; Yoshimura, Yoshitaka*; Narumi, Issei; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Fujisaki, Kenta*; Kawaguchi, Yuko*; Yang, Y.*; Fushimi, Hidehiko*; Hashimoto, Hirofumi*; Yamashita, Masamichi*; Yano, Hajime*; Okudaira, Kyoko*; Hayashi, Nobuhiro*; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Yang, Y.*; Sugino, Tomohiro*; Kawaguchi, Yuko*; Itahashi, Shiho*; Fujisaki, Kenta*; Fushimi, Hidehiko*; Hasegawa, Sunao*; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; et al.
no journal, ,
Yokobori, Shinichi*; Yang, Y.*; Sugino, Tomohiro*; Kawaguchi, Yuko*; Fushimi, Hidehiko*; Narumi, Issei; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; Kawai, Hideyuki*; Kobayashi, Kensei*; et al.
no journal, ,
Takayama, Reona*; Oe, Kazuhiro*; Komori, Yukiko*; Fujisawa, Hiroyuki*; Kuriyama, Ai*; Kikutani, Yuki*; Kikunaga, Hidetoshi*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; et al.
no journal, ,
The extraction behavior of trivalent actinides (Am, Cm, Cf, Es, and Fm) into di(2-ethylhexyl)phosphoric acid, HDEHP, in benzene from HNO was studied together with lanthanides. The extraction constants,
was studied together with lanthanides. The extraction constants, 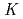
 values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of
values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of  Am,
Am,  Cm,
Cm,  Cf, and
Cf, and 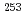 Es, respectively, while that of Fm was measured with
Es, respectively, while that of Fm was measured with 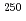 Fm which was produced in the
Fm which was produced in the  U(
U(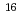 O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The
O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The 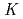
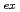 values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the
values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the 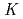
 of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
Mori, Takero; Sotsu, Masutake; Imaizumi, Yuya; Yoshimura, Kazuo; Fukano, Yoshitaka
no journal, ,
no abstracts in English
Kawaguchi, Yuko*; Sugino, Tomohiro*; Yang, Y.*; Takahashi, Yuta*; Yoshimura, Yoshitaka*; Tsuji, Takashi*; Kobayashi, Kensei*; Tabata, Makoto*; Hashimoto, Hirofumi*; Narumi, Issei; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Yang, Y.*; Kawaguchi, Yuko*; Sugino, Tomohiro*; Takahashi, Yuta*; Narumi, Issei; Takahashi, Yuichi*; Hayashi, Nobuhiro*; Yoshimura, Yoshitaka*; Tabata, Makoto*; et al.
no journal, ,
no abstracts in English
Sugino, Tomohiro*; Yokobori, Shinichi*; Yang, Y.*; Kawaguchi, Yuko*; Hasegawa, Sunao*; Hashimoto, Hirofumi*; Imai, Eiichi*; Okudaira, Kyoko*; Kawai, Hideyuki*; Tabata, Makoto*; et al.
no journal, ,
Yokobori, Shinichi*; Yang, Y.*; Sugino, Tomohiro*; Kawaguchi, Yuko*; Takahashi, Yuta*; Narumi, Issei; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; Imai, Eiichi*; Kawai, Hideyuki*; et al.
no journal, ,