Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Morita, Keisuke; Kitatsuji, Yoshihiro; Ito, Keisuke*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 28(2), p.121 - 131, 2021/00
Times Cited Count:2 Percentile:8.76(Chemistry, Multidisciplinary)High concentration of Cs is present in high-level radioactive waste. It is well-known that Cs is an alkali element and difficult to extract completely into an organic phase. Crown ether compounds are widely available for Cs extractants; DtBuDB18C6 (di- -butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as
-butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as  -dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios (
-dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios ( (Cs)), closely to 10.
(Cs)), closely to 10.
Sasaki, Yuji; Saeki, Morihisa*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 26(1), p.21 - 34, 2019/06
Times Cited Count:6 Percentile:21.71(Chemistry, Multidisciplinary)Three tridentate extractants and three masking reagents including O, N, and S donors have been developed and their properties are compared and discussed. The extractants are termed as tetraoctyl-diglycolamide (TODGA), methylimino-dioctylacetamide (MIDOA) and tetraoctyl-thiodiglycolamide (TDGA(C8)) and masking agents have the same central frame but with short alkyl chain. The results of the present study indicate that TODGA can extract mainly hard acid metals belonging groups 2-4,13-15 in periodic table, MIDOA can extract soft acid metals and oxyanions (groups 5-10, 16), and TDGA can extract soft acid metals (groups 10-11). Some spectrophotometric studies (UV, IR, and NMR) indicate the stoichiometry and effect of donor atoms for metal-complexation. The Hf values, the heat generation during complex formation, obtained by chemical calculation by DFT theory show the reverse-correlation with their extraction ability.
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo*; Yoshizuka, Kazuharu*
Proceedings of 21st International Solvent Extraction Conference (ISEC 2017) (Internet), p.131 - 134, 2017/11
Three tridentate extractants including soft and hard donor has been developed and examined. The extractants are termed as 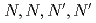 -tetraoctyl-diglycolamide (TODGA), methylimino-
-tetraoctyl-diglycolamide (TODGA), methylimino-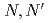 -dioctylacetamide (MIDOA) and
-dioctylacetamide (MIDOA) and 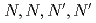 -tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
-tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo; Yoshizuka, Kazuharu*
Hydrometallurgy, 169, p.576 - 584, 2017/05
Times Cited Count:16 Percentile:55.22(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (TDGA), is analogous structure to tetraoctyl-diglycolamide (TODGA) and methylimino-dioctylacetamide (MIDOA), but with sulfur donor instead of ether oxygen or nitrogen atoms of TODGA or MIDOA. From the present work, TDGA can extract silver, palladium, gold, and mercury from acidic solutions to n-dodecane. In addition to these results, the distribution ratios of hard and soft acid metals by using TDGA, TODGA, and MIDOA are compared, where the metal-complexations with each donor atom are investigated. 1H-NMR and IR studies for the metal-TDGA complexes indicate the role on donor atoms, S and N, of TDGA.
 -tetraoctyl-thiodiglycolamide
-tetraoctyl-thiodiglycolamideSasaki, Yuji; Suzuki, Tomoya; Morita, Keisuke; Yoshizuka, Kazuharu*
Hydrometallurgy, 159, p.107 - 109, 2016/01
Times Cited Count:5 Percentile:24.71(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (S-DGA), is analogous structure to tetraoctyl-diglycolamide (TODGA), but with sulfur donor instead of ether oxygen of TODGA. S-DGA has unique property to extract silver from acidic solutions to n-dodecane with relatively high D values. We investigated the extraction behavior of Ag in various acids, HNO , H
, H SO
SO , and HClO
, and HClO .
.
Sasaki, Yuji; Kitatsuji, Yoshihiro; Hirata, Masaru; Kimura, Takaumi; Yoshizuka, Kazuharu*
Proceedings of International Solvent Extraction Conference "Solvent Extraction-Fundamentals to Industrial Applications" (ISEC 2008), p.745 - 750, 2008/09
The multidentate diamides are synthesized and examined for the actinide (An) extractions, bi- and tridentate extractants are focused in this work. The extraction of actinide was performed from 0.1-6M HNO to organic solvents. It was obvious that tetraalkyl-diglycolamide (DGA) derivatives, (methylimino)bis(N,N-dioctylacetamide) (MIDOA) and dimethyl-dioctyl-2-(3-oxapentadecane)-malonamide (DMDOOPDMA) have relatively high D values (D(Pu): more than 70). The following notable results using DGA extractants are obtained, (1) DGA with short alkyl chain give higher D values than those with long alkyl chain, (2) DGA with long alkyl chain have high solubility in n-dodecane. The molecular modeling with computational chemistry was also used to elucidate the effects of structural and electronic properties of the reagents on their different extractabilities.
to organic solvents. It was obvious that tetraalkyl-diglycolamide (DGA) derivatives, (methylimino)bis(N,N-dioctylacetamide) (MIDOA) and dimethyl-dioctyl-2-(3-oxapentadecane)-malonamide (DMDOOPDMA) have relatively high D values (D(Pu): more than 70). The following notable results using DGA extractants are obtained, (1) DGA with short alkyl chain give higher D values than those with long alkyl chain, (2) DGA with long alkyl chain have high solubility in n-dodecane. The molecular modeling with computational chemistry was also used to elucidate the effects of structural and electronic properties of the reagents on their different extractabilities.
Hirata, Masaru; Kimura, Takaumi; Yoshizuka, Kazuharu*
no journal, ,
no abstracts in English
Hirata, Masaru; Kimura, Takaumi; Yoshizuka, Kazuharu*
no journal, ,
no abstracts in English
Sasaki, Yuji; Yoshizuka, Kazuharu*
no journal, ,
It is important to study the solvent extraction of Cs, therefore the study on the diluent for the crown compounds is necessary. In this work, we investigate the physical properties of diluents and Cs extraction using the extraction solvents. The results indicate that distribution of Cs is higher when diluent having higher dielectric constant is used. Furthermore, the results using 2-nonanone, which have high dielectric constant, give the strong dependence of D(Cs) on nitric acid concentration.
Inoue, Katsutoshi*; Oto, Keisuke*; Yoshizuka, Kazuharu*; Naganawa, Hirochika; Tachimori, Shoichi*
no journal, ,
Lipophilic chitosan containing functional groups of dithiocarbamate (DTC-lipophilic chitosan) was prepared and tested for the selective solvent extraction of minor actinides (MA) from large excess of lanthanides in dilute nitric acid solution. This reagent was found to be soluble not only in chloroform and toluene but also in kerosene, which is very favorable for practical purposes. Although solid chitosan gel chemically modified with functional groups of dithiocarbamate prepared according to the conventional method had exhibited no selectivity to MA over lanthanides, DTC-lipophilic chitosan exhibited high selectivity to americium over europium; i.e., the separation factor of Am/Eu was as high as 1227 in the extraction at pH = 4. In addition, it exhibited the same separation factor at the second solvent extraction test carried out one week after the first test, suggesting that it is chemically stable at least for one week contrary to Cyanex 301 and DPTP.
Yoshizuka, Kazuharu*; Hirata, Masaru; Kimura, Takaumi
no journal, ,
no abstracts in English