Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Baccou, J.*; Glantz, T.*; Ghione, A.*; Sargentini, L.*; Fillion, P.*; Damblin, G.*; Sueur, R.*; Iooss, B.*; Fang, J.*; Liu, J.*; et al.
Nuclear Engineering and Design, 421, p.113035_1 - 113035_16, 2024/05
 neutron diffraction
neutron diffractionZhou, Y.*; Song, W.*; Zhang, F.*; Wu, Y.*; Lei, Z.*; Jiao, M.*; Zhang, X.*; Dong, J.*; Zhang, Y.*; Yang, M.*; et al.
Journal of Alloys and Compounds, 971, p.172635_1 - 172635_7, 2024/01
Times Cited Count:0 Percentile:0(Chemistry, Physical)Zhang, A.*; Deng, K.*; Sheng, J.*; Liu, P.*; Kumar, S.*; Shimada, Kenya*; Jiang, Z.*; Liu, Z.*; Shen, D.*; Li, J.*; et al.
Chinese Physics Letters, 40(12), p.126101_1 - 126101_8, 2023/12
Times Cited Count:1 Percentile:0(Physics, Multidisciplinary)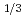 TaS
TaS
Park, P.*; Cho, W.*; Kim, C.*; An, Y.*; Kang, Y.-G.*; Avdeev, M.*; Sibille, R.*; Iida, Kazuki*; Kajimoto, Ryoichi; Lee, K. H.*; et al.
Nature Communications (Internet), 14, p.8346_1 - 8346_9, 2023/12
Times Cited Count:0 Percentile:0(Multidisciplinary Sciences) neutron diffraction study on the deformation behavior of the plastic inorganic semiconductor Ag
neutron diffraction study on the deformation behavior of the plastic inorganic semiconductor Ag S
SWang, Y.*; Gong, W.; Kawasaki, Takuro; Harjo, S.; Zhang, K.*; Zhang, Z. D.*; Li, B.*
Applied Physics Letters, 123(1), p.011903_1 - 011903_6, 2023/07
Times Cited Count:1 Percentile:54.89(Physics, Applied)Ao, N.*; Zhang, H.*; Xu, H. H.*; Wu, S. C.*; Liu, D.*; Xu, P. G.; Su, Y. H.; Kang, Q. H.*; Kang, G. Z.*
Engineering Fracture Mechanics, 281, p.109166_1 - 109166_14, 2023/03
Times Cited Count:4 Percentile:85.05(Mechanics)Jiang, X.*; Hattori, Takanori; Xu, X.*; Li, M.*; Yu, C.*; Yu, D.*; Mole, R.*; Yano, Shinichiro*; Chen, J.*; He, L.*; et al.
Materials Horizons, 10(3), p.977 - 982, 2023/03
Times Cited Count:5 Percentile:87.86(Chemistry, Multidisciplinary)As a promising environment-friendly alternative to current vapor-compression refrigeration, solid-state refrigeration based on the barocaloric effect has been attracting world wide attention. Generally, both phases in which a barocaloric effect occurs are present at ambient pressure. Here, instead, we demonstrate that KPF exhibits a colossal barocaloric effect due to the creation of a high-pressure rhombohedral phase. The phase diagram is constructed based on pressure-dependent calorimetric, Raman scattering, and neutron diffraction measurements. The present study is expected to provide an alternative routine to colossal barocaloric effects through the creation of a high-pressure phase.
exhibits a colossal barocaloric effect due to the creation of a high-pressure rhombohedral phase. The phase diagram is constructed based on pressure-dependent calorimetric, Raman scattering, and neutron diffraction measurements. The present study is expected to provide an alternative routine to colossal barocaloric effects through the creation of a high-pressure phase.
Chen, J.*; Yamamoto, Kei; Zhang, J.*; Ma, J.*; Wang, H.*; Sun, Y.*; Chen, M.*; Ma, J.*; Liu, S.*; Gao, P.*; et al.
Physical Review Applied (Internet), 19(2), p.024046_1 - 024046_9, 2023/02
Times Cited Count:4 Percentile:90.23(Physics, Applied)Liss, K.-D.*; Xu, P. G.; Shiro, Ayumi*; Zhang, S. Y.*; Yukutake, Eitaro*; Shobu, Takahisa; Akita, Koichi*
Advanced Engineering Materials, 9 Pages, 2023/00
Liu, X. J.*; Xu, P. G.; Shiro, Ayumi*; Zhang, S. Y.*; Shobu, Takahisa; Yukutake, Eitaro*; Akita, Koichi*; Zolotoyabko, E.*; Liss, K.-D.*
Journal of Materials Science, 57(46), p.21446 - 21459, 2022/12
Times Cited Count:3 Percentile:41.53(Materials Science, Multidisciplinary)Zhang, J.*; Kuang, L.*; Mou, Z.*; Kondo, Toshiaki*; Koarashi, Jun; Atarashi-Andoh, Mariko; Li, Y.*; Tang, X.*; Wang, Y.-P.*; Pe uelas, J.*; et al.
uelas, J.*; et al.
Plant and Soil, 481(1-2), p.349 - 365, 2022/12
Times Cited Count:3 Percentile:22.98(Agronomy) isomer in
isomer in 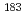 Hg and
Hg and  (
(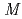 2) systematics of neutron transitions across the nuclear chart
2) systematics of neutron transitions across the nuclear chartHuang, H.*; Zhang, W. Q.*; Andreyev, A. N.; Liu, Z.*; Seweryniak, D.*; Li, Z. H.*; Guo, C. Y.*; Barzakh, A. E.*; Van Duppen, P.*; Andel, B.*; et al.
Physics Letters B, 833, p.137345_1 - 137345_8, 2022/10
Times Cited Count:0 Percentile:0.02(Astronomy & Astrophysics)Liu, B.*; Feng, R.*; Busch, M.*; Wang, S.*; Wu, H.*; Liu, P.*; Gu, J.*; Bahadoran, A.*; Matsumura, Daiju; Tsuji, Takuya; et al.
ACS Nano, 16(9), p.14121 - 14133, 2022/09
Times Cited Count:49 Percentile:98.33(Chemistry, Multidisciplinary)Khalil, A. M. E.*; Han, L.*; Maamoun, I.; Tabish, T. A.*; Chen, Y.*; Eljamal, O.*; Zhang, S.*; Butler, D.*; Memon, F. A.*
Advanced Sustainable Systems (Internet), 6(8), p.2200016_1 - 2200016_16, 2022/08
Times Cited Count:2 Percentile:29.01(Green & Sustainable Science & Technology) Bi and
Bi and  Po
PoZhang, W. Q.*; Andreyev, A. N.; Liu, Z.*; Seweryniak, D.*; Huang, H.*; 37 of others*
Physical Review C, 106(2), p.024317_1 - 024317_11, 2022/08
Times Cited Count:1 Percentile:33.4(Physics, Nuclear)Walter, H.*; Colonna, M.*; Cozma, D.*; Danielewicz, P.*; Ko, C. M.*; Kumar, R.*; Ono, Akira*; Tsang, M. Y. B*; Xu, J.*; Zhang, Y.-X.*; et al.
Progress in Particle and Nuclear Physics, 125, p.103962_1 - 103962_90, 2022/07
Times Cited Count:48 Percentile:96.94(Physics, Nuclear)Transport models are the main method to obtain physics information on the nuclear equation of state and in-medium properties of particles from low to relativistic-energy heavy-ion collisions. The Transport Model Evaluation Project (TMEP) has been pursued to test the robustness of transport model predictions to reach consistent conclusions from the same type of physical model. To this end, calculations under controlled conditions of physical input and set-up were performed by the various participating codes. These included both calculations of nuclear matter in a periodic box, which test individual ingredients of a transport code, and calculations of complete collisions of heavy ions. Over the years, five studies were performed within this project. They show, on one hand, that in box calculations the differences between the codes can be well understood and a convergence of the results can be reached. These studies also highlight the systematic differences between the two families of transport codes, known under the names of Boltzmann-Uehling-Uhlenbeck (BUU) and Quantum Molecular Dynamics (QMD) type codes. On the other hand, there still exist substantial differences when these codes are applied to real heavy-ion collisions. The results of transport simulations of heavy-ion collisions will have more significance if codes demonstrate that they can verify benchmark calculations such as the ones studied in these evaluations.
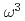 scaling of the vibrational density of states in quasi-2D nanoconfined solids
scaling of the vibrational density of states in quasi-2D nanoconfined solidsYu, Y.*; Yang, C.*; Baggioli, M.*; Phillips, A. E.*; Zaccone, A.*; Zhang, L.*; Kajimoto, Ryoichi; Nakamura, Mitsutaka; Yu, D.*; Hong, L.*
Nature Communications (Internet), 13, p.3649_1 - 3649_10, 2022/06
Times Cited Count:8 Percentile:81.66(Multidisciplinary Sciences)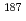 Pb
PbZhang, W. Q.*; Andreyev, A. N.; Liu, Z.*; Seweryniak, D.*; Huang, H.*; Li, Z. H.*; Li, J. G.*; Guo, C. Y.*; 34 of others*
Physics Letters B, 829, p.137129_1 - 137129_7, 2022/06
Times Cited Count:4 Percentile:76.34(Astronomy & Astrophysics)Gatera, A.*; Belmans, J.*; Boussa, S.*; Davin, F.*; De Cock, W.*; De Florio, V.*; Doucet, F.*; Parez, L.*; Pompon, F.*; Ponton, A.*; et al.
Proceedings of 64th ICFA Advanced Beam Dynamics Workshop on High Intensity and High Brightness Hadron Beams (HB2021), p.186 - 190, 2022/04
Wei, D.*; Wang, L.*; Zhang, Y.*; Gong, W.; Tsuru, Tomohito; Lobzenko, I.; Jiang, J.*; Harjo, S.; Kawasaki, Takuro; Bae, J. W.*; et al.
Acta Materialia, 225, p.117571_1 - 117571_16, 2022/02
Times Cited Count:59 Percentile:99.75(Materials Science, Multidisciplinary)