Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Li, F.*; Tang, X.*; Fei, Y.*; Zhang, J.*; Liu, J.*; Lang, P.*; Che, G.*; Zhao, Z.*; Zheng, Y.*; Fang, Y.*; et al.
Journal of the American Chemical Society, 147(17), p.14054 - 14059, 2025/04
Times Cited Count:1 Percentile:0.00(Chemistry, Multidisciplinary)We synthesized a crystalline graphane nanoribbon (GANR) via pressure-induced polymerization of 2,2'-bipyrazine (BPZ). By performing Rietveld refinement of in situ neutron diffraction data, nuclear magnetic resonance spectroscopy, infrared spectra, and theoretical calculation, we found that BPZ experienced Diels-Alder polymerization between the 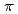
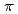 stacked aromatic rings, and formed extended boat-GANR structures with exceptional long-range order. The unreacted -C=N- groups bridge the two ends of the boat, and ready for further functionalization. The GANR has a bandgap of 2.25 eV, with booming photoelectric response (
stacked aromatic rings, and formed extended boat-GANR structures with exceptional long-range order. The unreacted -C=N- groups bridge the two ends of the boat, and ready for further functionalization. The GANR has a bandgap of 2.25 eV, with booming photoelectric response (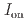 /
/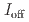 =18.8). Our work highlights that the high-pressure topochemical polymerization is a promising method for the precise synthesis of graphane with specific structure and desired properties.
=18.8). Our work highlights that the high-pressure topochemical polymerization is a promising method for the precise synthesis of graphane with specific structure and desired properties.
Rajeev, H. S.*; Hu, X.*; Chen, W.-L.*; Zhang, D.*; Chen, T.*; Kofu, Maiko*; Kajimoto, Ryoichi; Nakamura, Mitsutaka; Chen, A. Z.*; Johnson, G. C.*; et al.
Journal of the Physical Society of Japan, 94(3), p.034602_1 - 034602_14, 2025/03
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary) neutron imaging and diffraction analysis revealing spatial lithiation phase evolution in an ultra-thick graphite electrode
neutron imaging and diffraction analysis revealing spatial lithiation phase evolution in an ultra-thick graphite electrodeStrobl, M.*; Baur, M. E.*; Samothrakitis, S.*; Molamud, F.*; Zhang, X.*; Tung, P. K. M.*; Schmidt, S.*; Woracek, R.*; Lee, J.*; Kiyanagi, Ryoji; et al.
Advanced Energy Materials, p.2405238_1 - 2405238_9, 2025/01
Times Cited Count:0Yang, X.*; Che, G.*; Wang, Y.*; Zhang, P.*; Tang, X.*; Lang, P.*; Gao, D.*; Wang, X.*; Wang, Y.*; Hattori, Takanori; et al.
Nano Letters, 25(3), p.1028 - 1035, 2025/01
Times Cited Count:1 Percentile:84.76(Chemistry, Multidisciplinary)Saturated sp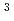 -carbon nanothreads (CNTh) have garnered significant interest due to their predicted high Young's modulus and thermal conductivity. While the incorporation of heteroatoms into the central ring has been shown to influence the formation of CNTh and yield chemically homogeneous products, the impact of pendant groups on the polymerization process remains underexplored. In this study, we investigate the pressure-induced polymerization of phenol, revealing two phase transitions occurring below 0.5 and 4 GPa. Above 20 GPa, phenol polymerizes into degree-4 CNThs featuring hydroxyl and carbonyl groups. Hydrogen transfer of hydroxyl groups was found to hinder the formation of degree-6 nanothreads. Our findings highlight the crucial role of the hydroxyl group in halting further intracolumn polymerization and offer valuable insights for future mechanism research and nanomaterial synthesis.
-carbon nanothreads (CNTh) have garnered significant interest due to their predicted high Young's modulus and thermal conductivity. While the incorporation of heteroatoms into the central ring has been shown to influence the formation of CNTh and yield chemically homogeneous products, the impact of pendant groups on the polymerization process remains underexplored. In this study, we investigate the pressure-induced polymerization of phenol, revealing two phase transitions occurring below 0.5 and 4 GPa. Above 20 GPa, phenol polymerizes into degree-4 CNThs featuring hydroxyl and carbonyl groups. Hydrogen transfer of hydroxyl groups was found to hinder the formation of degree-6 nanothreads. Our findings highlight the crucial role of the hydroxyl group in halting further intracolumn polymerization and offer valuable insights for future mechanism research and nanomaterial synthesis.
Xu, J.*; Lang, P.*; Liang, S.*; Zhang, J.*; Fei, Y.*; Wang, Y.*; Gao, D.*; Hattori, Takanori; Abe, Jun*; Dong, X.*; et al.
Journal of Physical Chemistry Letters (Internet), p.2445 - 2451, 2025/00
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)The Alder-ene reaction is a chemical reaction between an alkene with an allylic hydrogen, and it provides an efficient method to construct the C-C bond. Traditionally, this reaction requires catalysts, high temperatures, or photocatalysis. In this study, we reported a high-pressure-induced solid-state Alder-ene reaction of 1-hexene at room temperature without a catalyst. 1-Hexene crystallizes at 4.3 GPa and polymerizes at 18 GPa, forming olefins. By exploring gas chromatography-mass spectrometry, we discovered that 1-hexene generates dimeric products through the Alder-ene reaction under high pressures. The in situ neutron diffraction shows that the reaction process did not obey the topochemical rule. A six-membered ring transition state including one C-H  bond and two alkene
bond and two alkene 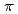 bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
Liu, P.-F.*; Li, X.*; Li, J.*; Zhu, J.*; Tong, Z.*; Kofu, Maiko*; Nirei, Masami; Xu, J.*; Yin, W.*; Wang, F.*; et al.
National Science Review, 11(12), p.nwae216_1 - nwae216_10, 2024/12
Times Cited Count:13 Percentile:94.32(Multidisciplinary Sciences)Zhang, Y.-J.*; Umeda, Takemasa*; Morooka, Satoshi; Harjo, S.; Miyamoto, Goro*; Furuhara, Tadashi*
Metallurgical and Materials Transactions A, 55(10), p.3921 - 3936, 2024/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Matsuura, Masato*; Zhang, J.*; Kamimura, Yasushi*; Kofu, Maiko; Edagawa, Keiichi*
Physical Review Letters, 133(13), p.136101_1 - 136101_5, 2024/09
Times Cited Count:1 Percentile:43.84(Physics, Multidisciplinary)Igarashi, Junya*; Ninomiya, Kazuhiko*; Zheng, J.*; Zhang, Z. J.*; Fukuda, Miho*; Aono, Tatsuo*; Minowa, Haruka*; Yoshikawa, Hideki*; Sueki, Keisuke*; Satou, Yukihiko; et al.
Environmental Science & Technology, 58(33), p.14823 - 14830, 2024/08
Times Cited Count:0 Percentile:0.00(Engineering, Environmental)Fang, W.*; Liu, C.*; Zhang, J.*; Xu, P. G.; Peng, T.*; Liu, B.*; Morooka, Satoshi; Yin, F.*
Scripta Materialia, 249, p.116046_1 - 116046_6, 2024/08
Times Cited Count:2 Percentile:57.76(Nanoscience & Nanotechnology)Zhang, Z.*; Hattori, Takanori; Song, R.*; Yu, D.*; Mole, R.*; Chen, J.*; He, L.*; Zhang, Z.*; Li, B.*
Journal of Applied Physics, 136(3), p.035105_1 - 035105_8, 2024/07
Times Cited Count:2 Percentile:35.22(Physics, Applied)Solid-state refrigeration using barocaloric materials is environmentally friendly and highly efficient, making it a subject of global interest over the past decade. Here, we report giant barocaloric effects in sodium hexafluorophosphate (NaPF ) and sodium hexafluoroarsenate (NaAsF
) and sodium hexafluoroarsenate (NaAsF ) that both undergo a cubic-to-rhombohedral phase transition near room temperature. We have determined that the low-temperature phase structure of NaPF
) that both undergo a cubic-to-rhombohedral phase transition near room temperature. We have determined that the low-temperature phase structure of NaPF is a rhombohedral structure with space group R
is a rhombohedral structure with space group R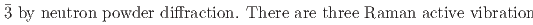 and NaAsF
and NaAsF , i.e., F
, i.e., F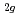 , E
, E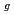 , and A
, and A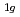 . The phase transition temperature varies with pressure at a rate of dT
. The phase transition temperature varies with pressure at a rate of dT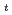 /dP = 250 and 310 K/GPa for NaPF
/dP = 250 and 310 K/GPa for NaPF and NaAsF
and NaAsF . The pressure-induced entropy changes of NaPF
. The pressure-induced entropy changes of NaPF and NaAsF
and NaAsF are determined to be around 45.2 and 35.6J kg
are determined to be around 45.2 and 35.6J kg K
K , respectively. The saturation driving pressure is about 40 MPa. The pressure-dependent neutron powder diffraction suggests that the barocaloric effects are related to the pressure-induced cubic-to-rhombohedral phase transitions.
, respectively. The saturation driving pressure is about 40 MPa. The pressure-dependent neutron powder diffraction suggests that the barocaloric effects are related to the pressure-induced cubic-to-rhombohedral phase transitions.
Zhou, L.*; Zhang, H.*; Qin, T. Y.*; Hu, F. F.*; Xu, P. G.; Ao, N.*; Su, Y. H.; He, L. H.*; Li, X. H.*; Zhang, J. R.*; et al.
Metallurgical and Materials Transactions A, 55(7), p.2175 - 2185, 2024/07
Times Cited Count:3 Percentile:70.74(Materials Science, Multidisciplinary)Wang, S.*; Wang, J.*; Zhang, S.*; Wei, D.*; Chen, Y.*; Rong, X.*; Gong, W.; Harjo, S.; Liu, X.*; Jiao, Z.*; et al.
Journal of Materials Science & Technology, 185, p.245 - 258, 2024/06
Times Cited Count:17 Percentile:98.00(Materials Science, Multidisciplinary)
Liao, J.*; Huang, Z.*; Shangguan, Y.*; Zhang, B.*; Cheng, S.*; Xu, H.*; Kajimoto, Ryoichi; Kamazawa, Kazuya*; Bao, S.*; Wen, J.*
Physical Review B, 109(22), p.224411_1 - 224411_10, 2024/06
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Baccou, J.*; Glantz, T.*; Ghione, A.*; Sargentini, L.*; Fillion, P.*; Damblin, G.*; Sueur, R.*; Iooss, B.*; Fang, J.*; Liu, J.*; et al.
Nuclear Engineering and Design, 421, p.113035_1 - 113035_16, 2024/05
Times Cited Count:6 Percentile:96.49(Nuclear Science & Technology)Guo, B.*; Chen, H.*; Chong, Y.*; Mao, W.; Harjo, S.; Gong, W.; Zhang, Z.*; Jonas, J. J.*; Tsuji, Nobuhiro*
Acta Materialia, 268, p.119780_1 - 119780_11, 2024/04
Times Cited Count:10 Percentile:94.16(Materials Science, Multidisciplinary) -MgAgSb
-MgAgSbLi, J.*; Li, X.*; Zhang, Y.*; Zhu, J.*; Zhao, E.*; Kofu, Maiko; Nakajima, Kenji; Avdeev, M.*; Liu, P.-F.*; Sui, J.*; et al.
Applied Physics Reviews (Internet), 11(1), p.011406_1 - 011406_8, 2024/03
Times Cited Count:11 Percentile:89.71(Physics, Applied)Li, X.*; Zhu, R.*; Xin, J.*; Luo, M.*; Shang, S.-L.*; Liu, Z.-K.*; Yin, C.*; Funakoshi, Kenichi*; Dippenaar, R. J.*; Higo, Yuji*; et al.
CALPHAD; Computer Coupling of Phase Diagrams and Thermochemistry, 84, p.102641_1 - 102641_6, 2024/03
Times Cited Count:0 Percentile:0.00(Thermodynamics) neutron diffraction
neutron diffractionZhou, Y.*; Song, W.*; Zhang, F.*; Wu, Y.*; Lei, Z.*; Jiao, M.*; Zhang, X.*; Dong, J.*; Zhang, Y.*; Yang, M.*; et al.
Journal of Alloys and Compounds, 971, p.172635_1 - 172635_7, 2024/01
Times Cited Count:3 Percentile:23.52(Chemistry, Physical)Zhang, A.*; Deng, K.*; Sheng, J.*; Liu, P.*; Kumar, S.*; Shimada, Kenya*; Jiang, Z.*; Liu, Z.*; Shen, D.*; Li, J.*; et al.
Chinese Physics Letters, 40(12), p.126101_1 - 126101_8, 2023/12
Times Cited Count:11 Percentile:85.96(Physics, Multidisciplinary)