Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Rajeev, H. S.*; Hu, X.*; Chen, W.-L.*; Zhang, D.*; Chen, T.*; Kofu, Maiko*; Kajimoto, Ryoichi; Nakamura, Mitsutaka; Chen, A. Z.*; Johnson, G. C.*; et al.
Journal of the Physical Society of Japan, 94(3), p.034602_1 - 034602_14, 2025/03
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary)Brumm, S.*; Gabrielli, F.*; Sanchez Espinoza, V.*; Stakhanova, A.*; Groudev, P.*; Petrova, P.*; Vryashkova, P.*; Ou, P.*; Zhang, W.*; Malkhasyan, A.*; et al.
Annals of Nuclear Energy, 211, p.110962_1 - 110962_16, 2025/02
Times Cited Count:6 Percentile:94.85(Nuclear Science & Technology) neutron imaging and diffraction analysis revealing spatial lithiation phase evolution in an ultra-thick graphite electrode
neutron imaging and diffraction analysis revealing spatial lithiation phase evolution in an ultra-thick graphite electrodeStrobl, M.*; Baur, M. E.*; Samothrakitis, S.*; Molamud, F.*; Zhang, X.*; Tung, P. K. M.*; Schmidt, S.*; Woracek, R.*; Lee, J.*; Kiyanagi, Ryoji; et al.
Advanced Energy Materials, p.2405238_1 - 2405238_9, 2025/01
Times Cited Count:0Xu, J.*; Lang, P.*; Liang, S.*; Zhang, J.*; Fei, Y.*; Wang, Y.*; Gao, D.*; Hattori, Takanori; Abe, Jun*; Dong, X.*; et al.
Journal of Physical Chemistry Letters (Internet), p.2445 - 2451, 2025/00
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)The Alder-ene reaction is a chemical reaction between an alkene with an allylic hydrogen, and it provides an efficient method to construct the C-C bond. Traditionally, this reaction requires catalysts, high temperatures, or photocatalysis. In this study, we reported a high-pressure-induced solid-state Alder-ene reaction of 1-hexene at room temperature without a catalyst. 1-Hexene crystallizes at 4.3 GPa and polymerizes at 18 GPa, forming olefins. By exploring gas chromatography-mass spectrometry, we discovered that 1-hexene generates dimeric products through the Alder-ene reaction under high pressures. The in situ neutron diffraction shows that the reaction process did not obey the topochemical rule. A six-membered ring transition state including one C-H  bond and two alkene
bond and two alkene  bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
Zhang, Y.-J.*; Umeda, Takemasa*; Morooka, Satoshi; Harjo, S.; Miyamoto, Goro*; Furuhara, Tadashi*
Metallurgical and Materials Transactions A, 55(10), p.3921 - 3936, 2024/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Ying, H.*; Yang, X.*; He, H.*; Yan, A.*; An, K.*; Ke, Y.*; Wu, Z.*; Tang, S.*; Zhang, Z.*; Dong, H.*; et al.
Scripta Materialia, 250, p.116181_1 - 116181_7, 2024/09
Times Cited Count:2 Percentile:36.18(Nanoscience & Nanotechnology)Qin, T. Y.*; Hu, F. F.*; Xu, P. G.; Zhang, H.*; Zhou, L.*; Ao, N.*; Su, Y. H.; Shobu, Takahisa; Wu, S. C.*
International Journal of Fatigue, 185, p.108336_1 - 108336_13, 2024/08
Times Cited Count:9 Percentile:93.27(Engineering, Mechanical)Watanabe, Miku*; Miyamoto, Goro*; Zhang, Y.*; Morooka, Satoshi; Harjo, S.; Kobayashi, Yasuhiro*; Furuhara, Tadashi*
ISIJ International, 64(9), p.1464 - 1476, 2024/07
Times Cited Count:3 Percentile:57.76(Metallurgy & Metallurgical Engineering)Zhou, L.*; Zhang, H.*; Qin, T. Y.*; Hu, F. F.*; Xu, P. G.; Ao, N.*; Su, Y. H.; He, L. H.*; Li, X. H.*; Zhang, J. R.*; et al.
Metallurgical and Materials Transactions A, 55(7), p.2175 - 2185, 2024/07
Times Cited Count:3 Percentile:70.74(Materials Science, Multidisciplinary)Zeng, Z.*; Zhou, C.*; Zhou, H.*; Han, L.*; Chi, R.*; Li, K.*; Kofu, Maiko; Nakajima, Kenji; Wei, Y.*; Zhang, W.*; et al.
Nature Physics, 20(7), p.1097 - 1102, 2024/07
Times Cited Count:12 Percentile:94.31(Physics, Multidisciplinary)Wang, S.*; Wang, J.*; Zhang, S.*; Wei, D.*; Chen, Y.*; Rong, X.*; Gong, W.; Harjo, S.; Liu, X.*; Jiao, Z.*; et al.
Journal of Materials Science & Technology, 185, p.245 - 258, 2024/06
Times Cited Count:17 Percentile:98.00(Materials Science, Multidisciplinary)
Liao, J.*; Huang, Z.*; Shangguan, Y.*; Zhang, B.*; Cheng, S.*; Xu, H.*; Kajimoto, Ryoichi; Kamazawa, Kazuya*; Bao, S.*; Wen, J.*
Physical Review B, 109(22), p.224411_1 - 224411_10, 2024/06
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Baccou, J.*; Glantz, T.*; Ghione, A.*; Sargentini, L.*; Fillion, P.*; Damblin, G.*; Sueur, R.*; Iooss, B.*; Fang, J.*; Liu, J.*; et al.
Nuclear Engineering and Design, 421, p.113035_1 - 113035_16, 2024/05
Times Cited Count:6 Percentile:96.49(Nuclear Science & Technology)Guo, B.*; Chen, H.*; Chong, Y.*; Mao, W.; Harjo, S.; Gong, W.; Zhang, Z.*; Jonas, J. J.*; Tsuji, Nobuhiro*
Acta Materialia, 268, p.119780_1 - 119780_11, 2024/04
Times Cited Count:10 Percentile:94.16(Materials Science, Multidisciplinary)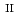 complex exhibiting intermolecular proton shifting coupled spin transition
complex exhibiting intermolecular proton shifting coupled spin transitionJi, T.*; Su, S.*; Wu, S.*; Hori, Yuta*; Shigeta, Yasuteru*; Huang, Y.*; Zheng, W.*; Xu, W.*; Zhang, X.*; Kiyanagi, Ryoji; et al.
Angewandte Chemie; International Edition, 63(25), p.e202404843_1 - e202404843_6, 2024/04
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Li, X.*; Zhu, R.*; Xin, J.*; Luo, M.*; Shang, S.-L.*; Liu, Z.-K.*; Yin, C.*; Funakoshi, Kenichi*; Dippenaar, R. J.*; Higo, Yuji*; et al.
CALPHAD; Computer Coupling of Phase Diagrams and Thermochemistry, 84, p.102641_1 - 102641_6, 2024/03
Times Cited Count:0 Percentile:0.00(Thermodynamics)Liss, K.-D.*; Xu, P. G.; Shiro, Ayumi*; Zhang, S. Y.*; Yukutake, Eitaro*; Shobu, Takahisa; Akita, Koichi*
Advanced Engineering Materials, 26(4), p.202300470_1 - 202300470_9, 2024/02
Times Cited Count:5 Percentile:47.86(Materials Science, Multidisciplinary)Zhang, Y.*; Marusawa, Kenji*; Kudo, Kohei*; Morooka, Satoshi; Harjo, S.; Miyamoto, Goro*; Furuhara, Tadashi*
ISIJ International, 64(2), p.245 - 256, 2024/01
Times Cited Count:2 Percentile:57.76(Metallurgy & Metallurgical Engineering) neutron diffraction
neutron diffractionZhou, Y.*; Song, W.*; Zhang, F.*; Wu, Y.*; Lei, Z.*; Jiao, M.*; Zhang, X.*; Dong, J.*; Zhang, Y.*; Yang, M.*; et al.
Journal of Alloys and Compounds, 971, p.172635_1 - 172635_7, 2024/01
Times Cited Count:3 Percentile:23.52(Chemistry, Physical) -Ti alloy
-Ti alloyZhang, B.*; Xin, S.*; Huang, M.*; Mao, W.; Jia, W.*; Li, Q.*; Li, S.*; Zhang, S.*; Mao, C.*
Materials Science & Engineering A, 890, p.145898_1 - 145898_7, 2024/01
Times Cited Count:0 Percentile:0.00(Nanoscience & Nanotechnology)A significant increase in the recovery strain of a high-Zr  -Ti alloy from 2.25 % to 5.5 % when decreasing the deformation temperature from 300 K to 77 K is reported in this study. It is found that the super-elasticity of this alloy is independent of the
-Ti alloy from 2.25 % to 5.5 % when decreasing the deformation temperature from 300 K to 77 K is reported in this study. It is found that the super-elasticity of this alloy is independent of the  -grain size at 77 K. The results reveal that a coarse-grained specimen exhibited approximately the same super-elasticity as its ultra-fine grain counterpart at 77 K. The relative easiness of deformation-induced martensitic transformation and dislocation slip was substantially changed at 77 K, with a strong suppression of dislocation slip, which overshadowed the effect of grain refinement on the super-elasticity.
-grain size at 77 K. The results reveal that a coarse-grained specimen exhibited approximately the same super-elasticity as its ultra-fine grain counterpart at 77 K. The relative easiness of deformation-induced martensitic transformation and dislocation slip was substantially changed at 77 K, with a strong suppression of dislocation slip, which overshadowed the effect of grain refinement on the super-elasticity.