Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Li, F.*; Tang, X.*; Fei, Y.*; Zhang, J.*; Liu, J.*; Lang, P.*; Che, G.*; Zhao, Z.*; Zheng, Y.*; Fang, Y.*; et al.
Journal of the American Chemical Society, 147(17), p.14054 - 14059, 2025/04
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)We synthesized a crystalline graphane nanoribbon (GANR) via pressure-induced polymerization of 2,2'-bipyrazine (BPZ). By performing Rietveld refinement of in situ neutron diffraction data, nuclear magnetic resonance spectroscopy, infrared spectra, and theoretical calculation, we found that BPZ experienced Diels-Alder polymerization between the 
 stacked aromatic rings, and formed extended boat-GANR structures with exceptional long-range order. The unreacted -C=N- groups bridge the two ends of the boat, and ready for further functionalization. The GANR has a bandgap of 2.25 eV, with booming photoelectric response (
stacked aromatic rings, and formed extended boat-GANR structures with exceptional long-range order. The unreacted -C=N- groups bridge the two ends of the boat, and ready for further functionalization. The GANR has a bandgap of 2.25 eV, with booming photoelectric response (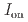 /
/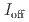 =18.8). Our work highlights that the high-pressure topochemical polymerization is a promising method for the precise synthesis of graphane with specific structure and desired properties.
=18.8). Our work highlights that the high-pressure topochemical polymerization is a promising method for the precise synthesis of graphane with specific structure and desired properties.
Zhang, X.-T.*; Xing, Y.-H.*; Yao, X.-P.*; Ominato, Yuya*; Zhang, L.*; Matsuo, Mamoru
Communications Physics (Internet), 8, p.103_1 - 103_8, 2025/03
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary) neutron imaging and diffraction analysis revealing spatial lithiation phase evolution in an ultra-thick graphite electrode
neutron imaging and diffraction analysis revealing spatial lithiation phase evolution in an ultra-thick graphite electrodeStrobl, M.*; Baur, M. E.*; Samothrakitis, S.*; Molamud, F.*; Zhang, X.*; Tung, P. K. M.*; Schmidt, S.*; Woracek, R.*; Lee, J.*; Kiyanagi, Ryoji; et al.
Advanced Energy Materials, p.2405238_1 - 2405238_9, 2025/01
Yang, X.*; Che, G.*; Wang, Y.*; Zhang, P.*; Tang, X.*; Lang, P.*; Gao, D.*; Wang, X.*; Wang, Y.*; Hattori, Takanori; et al.
Nano Letters, 25(3), p.1028 - 1035, 2025/01
Times Cited Count:1 Percentile:0.00(Chemistry, Multidisciplinary)Saturated sp -carbon nanothreads (CNTh) have garnered significant interest due to their predicted high Young's modulus and thermal conductivity. While the incorporation of heteroatoms into the central ring has been shown to influence the formation of CNTh and yield chemically homogeneous products, the impact of pendant groups on the polymerization process remains underexplored. In this study, we investigate the pressure-induced polymerization of phenol, revealing two phase transitions occurring below 0.5 and 4 GPa. Above 20 GPa, phenol polymerizes into degree-4 CNThs featuring hydroxyl and carbonyl groups. Hydrogen transfer of hydroxyl groups was found to hinder the formation of degree-6 nanothreads. Our findings highlight the crucial role of the hydroxyl group in halting further intracolumn polymerization and offer valuable insights for future mechanism research and nanomaterial synthesis.
-carbon nanothreads (CNTh) have garnered significant interest due to their predicted high Young's modulus and thermal conductivity. While the incorporation of heteroatoms into the central ring has been shown to influence the formation of CNTh and yield chemically homogeneous products, the impact of pendant groups on the polymerization process remains underexplored. In this study, we investigate the pressure-induced polymerization of phenol, revealing two phase transitions occurring below 0.5 and 4 GPa. Above 20 GPa, phenol polymerizes into degree-4 CNThs featuring hydroxyl and carbonyl groups. Hydrogen transfer of hydroxyl groups was found to hinder the formation of degree-6 nanothreads. Our findings highlight the crucial role of the hydroxyl group in halting further intracolumn polymerization and offer valuable insights for future mechanism research and nanomaterial synthesis.
Xu, J.*; Lang, P.*; Liang, S.*; Zhang, J.*; Fei, Y.*; Wang, Y.*; Gao, D.*; Hattori, Takanori; Abe, Jun*; Dong, X.*; et al.
Journal of Physical Chemistry Letters (Internet), p.2445 - 2451, 2025/00
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)The Alder-ene reaction is a chemical reaction between an alkene with an allylic hydrogen, and it provides an efficient method to construct the C-C bond. Traditionally, this reaction requires catalysts, high temperatures, or photocatalysis. In this study, we reported a high-pressure-induced solid-state Alder-ene reaction of 1-hexene at room temperature without a catalyst. 1-Hexene crystallizes at 4.3 GPa and polymerizes at 18 GPa, forming olefins. By exploring gas chromatography-mass spectrometry, we discovered that 1-hexene generates dimeric products through the Alder-ene reaction under high pressures. The in situ neutron diffraction shows that the reaction process did not obey the topochemical rule. A six-membered ring transition state including one C-H  bond and two alkene
bond and two alkene  bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
Ying, H.*; Yang, X.*; He, H.*; Yan, A.*; An, K.*; Ke, Y.*; Wu, Z.*; Tang, S.*; Zhang, Z.*; Dong, H.*; et al.
Scripta Materialia, 250, p.116181_1 - 116181_7, 2024/09
Times Cited Count:1 Percentile:41.92(Nanoscience & Nanotechnology)Zhou, L.*; Zhang, H.*; Qin, T. Y.*; Hu, F. F.*; Xu, P. G.; Ao, N.*; Su, Y. H.; He, L. H.*; Li, X. H.*; Zhang, J. R.*; et al.
Metallurgical and Materials Transactions A, 55(7), p.2175 - 2185, 2024/07
Times Cited Count:3 Percentile:75.40(Materials Science, Multidisciplinary)Baccou, J.*; Glantz, T.*; Ghione, A.*; Sargentini, L.*; Fillion, P.*; Damblin, G.*; Sueur, R.*; Iooss, B.*; Fang, J.*; Liu, J.*; et al.
Nuclear Engineering and Design, 421, p.113035_1 - 113035_16, 2024/05
Times Cited Count:6 Percentile:97.32(Nuclear Science & Technology) -MgAgSb
-MgAgSbLi, J.*; Li, X.*; Zhang, Y.*; Zhu, J.*; Zhao, E.*; Kofu, Maiko; Nakajima, Kenji; Avdeev, M.*; Liu, P.-F.*; Sui, J.*; et al.
Applied Physics Reviews (Internet), 11(1), p.011406_1 - 011406_8, 2024/03
Times Cited Count:7 Percentile:91.57(Physics, Applied)Lloveras, P.*; Zhang, Z.*; Zeng, M.*; Barrio, M.*; Kawakita, Yukinobu; Yu, D.*; Lin, S.*; Li, K.*; Moya, X.*; Tamarit, J.-L.*; et al.
Barocaloric Effects in the Solid State; Materials and methods, p.7_1 - 7_30, 2023/10
Times Cited Count:232 Percentile:99.37(Multidisciplinary Sciences)As Chapter 1 of the ebook entitled as "Barocaloric Effects in the Solid State", various plastic crystals (PC) showing colossal barocaloric (BC) effect are introduced. A method to determine the BC response in PCs, thermodynamic origin of BC effects, spectroscopic insights from quasi-elastic neutron scattering and application of PCs are explained.
 ; Bandgap narrowing, metallization, and remarkable enhancement of photoelectric activity
; Bandgap narrowing, metallization, and remarkable enhancement of photoelectric activityFang, Y.*; Kong, L.*; Wang, R.*; Zhang, Z.*; Li, Z.*; Wu, Y.*; Bu, K.*; Liu, X.*; Yan, S.*; Hattori, Takanori; et al.
Materials Today Physics (Internet), 34, p.101083_1 - 101083_7, 2023/05
Times Cited Count:8 Percentile:74.51(Materials Science, Multidisciplinary)The layered van der Waals halides are particularly sensitive to external pressure, suggesting a feasible route to pinpoint their structure with extraordinary behavior. However, a very sensitive pressure response usually lead to a detrimental phase transition and/or lattice distortion, making the approach of materials manipulation in a continuous manner remain challenging. Here, the extremely weak interlayer coupling and high tunability of layered RhI crystals are observed. A pressure-driven phase transition occurs at a moderate pressure of 5 GPa, interlinking to a change of layer stack mode. Strikingly, such a phase transition does not affect the tendency of quasi-linear bandgap narrowing, and a metallization with an ultra-broad tunability of 1.3 eV redshift is observed at higher pressures. Moreover, the carrier concentration increases by 4 orders of magnitude at 30 GPa, and the photocurrent enhances by 5 orders of magnitude at 7.8 GPa. These findings create new opportunities for exploring, tuning, and understanding the van der Waals halides by harnessing their unusual feature of a layered structure, which is promising for future devices based on materials-by-design that are atomically thin.
crystals are observed. A pressure-driven phase transition occurs at a moderate pressure of 5 GPa, interlinking to a change of layer stack mode. Strikingly, such a phase transition does not affect the tendency of quasi-linear bandgap narrowing, and a metallization with an ultra-broad tunability of 1.3 eV redshift is observed at higher pressures. Moreover, the carrier concentration increases by 4 orders of magnitude at 30 GPa, and the photocurrent enhances by 5 orders of magnitude at 7.8 GPa. These findings create new opportunities for exploring, tuning, and understanding the van der Waals halides by harnessing their unusual feature of a layered structure, which is promising for future devices based on materials-by-design that are atomically thin.
Xia, C.-J.*; Maruyama, Toshiki; Yasutake, Nobutoshi*; Tatsumi, Toshitaka*; Zhang, Y.-X.*
Physics Letters B, 839, p.137769_1 - 137769_5, 2023/04
Times Cited Count:2 Percentile:45.98(Astronomy & Astrophysics)Liu, X. J.*; Xu, P. G.; Shiro, Ayumi*; Zhang, S. Y.*; Shobu, Takahisa; Yukutake, Eitaro*; Akita, Koichi*; Zolotoyabko, E.*; Liss, K.-D.*
Journal of Materials Science, 57(46), p.21446 - 21459, 2022/12
Times Cited Count:5 Percentile:34.44(Materials Science, Multidisciplinary)Wang, Q.*; Hu, Q.*; Zhao, C.*; Yang, X.*; Zhang, T.*; Ilavsky, J.*; Kuzmenko, I.*; Ma, B.*; Tachi, Yukio
International Journal of Coal Geology, 261, p.104093_1 - 104093_15, 2022/09
Times Cited Count:11 Percentile:71.33(Energy & Fuels) decay of the 8
decay of the 8 isomer in
isomer in 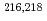 U
UZhang, M. M.*; Tian, Y. L.*; Wang, Y. S.*; Zhang, Z. Y.*; Gan, Z. G.*; Yang, H. B.*; Huang, M. H.*; Ma, L.*; Yang, C. L.*; Wang, J. G.*; et al.
Physical Review C, 106(2), p.024305_1 - 024305_6, 2022/08
Times Cited Count:5 Percentile:61.57(Physics, Nuclear)Walter, H.*; Colonna, M.*; Cozma, D.*; Danielewicz, P.*; Ko, C. M.*; Kumar, R.*; Ono, Akira*; Tsang, M. Y. B*; Xu, J.*; Zhang, Y.-X.*; et al.
Progress in Particle and Nuclear Physics, 125, p.103962_1 - 103962_90, 2022/07
Times Cited Count:88 Percentile:95.20(Physics, Nuclear)Transport models are the main method to obtain physics information on the nuclear equation of state and in-medium properties of particles from low to relativistic-energy heavy-ion collisions. The Transport Model Evaluation Project (TMEP) has been pursued to test the robustness of transport model predictions to reach consistent conclusions from the same type of physical model. To this end, calculations under controlled conditions of physical input and set-up were performed by the various participating codes. These included both calculations of nuclear matter in a periodic box, which test individual ingredients of a transport code, and calculations of complete collisions of heavy ions. Over the years, five studies were performed within this project. They show, on one hand, that in box calculations the differences between the codes can be well understood and a convergence of the results can be reached. These studies also highlight the systematic differences between the two families of transport codes, known under the names of Boltzmann-Uehling-Uhlenbeck (BUU) and Quantum Molecular Dynamics (QMD) type codes. On the other hand, there still exist substantial differences when these codes are applied to real heavy-ion collisions. The results of transport simulations of heavy-ion collisions will have more significance if codes demonstrate that they can verify benchmark calculations such as the ones studied in these evaluations.
Wang, X.*; Tang, X.*; Zhang, P.*; Wang, Y.*; Gao, D.*; Liu, J.*; Hui, K.*; Wang, Y.*; Dong, X.*; Hattori, Takanori; et al.
Journal of Physical Chemistry Letters (Internet), 12(50), p.12055 - 12061, 2021/12
Times Cited Count:14 Percentile:71.30(Chemistry, Physical)Substituted polyacetylene is expected to improve the chemical stability, physical properties, and additional functions of the polyacetylene backbones, but its diversity is very limited. Here, by applying external pressure on solid acetylenedicarboxylic acid, we report the first crystalline poly-dicarboxylacetylene with every carbon on the trans-polyacetylene backbone bonded to a carboxyl group, which is very hard to synthesize by traditional methods. This unique structure combines the extremely high content of carbonyl groups and high conductivity of a polyacetylene backbone, which exhibits a high specific capacity and excellent cycling/rate performance as a Li-ion battery (LIB) anode. We present a completely functionalized crystalline polyacetylene and provide a high-pressure solution for the synthesis of polymeric LIB materials and other polymeric materials with a high content of active groups.
Oyanagi, Koichi*; Gomez-Perez, J. M.*; Zhang, X.-P.*; Kikkawa, Takashi*; Chen, Y.*; Sagasta, E.*; Chuvilin, A.*; Hueso, L. E.*; Golovach, V. N.*; Sebastian Bergeret, F.*; et al.
Physical Review B, 104(13), p.134428_1 - 134428_14, 2021/10
Times Cited Count:18 Percentile:75.12(Materials Science, Multidisciplinary)Gao, D.*; Tang, X.*; Wang, X.*; Yang, X.*; Zhang, P.*; Che, G.*; Han, J.*; Hattori, Takanori; Wang, Y.*; Dong, X.*; et al.
Physical Chemistry Chemical Physics, 23(35), p.19503 - 19510, 2021/09
Times Cited Count:7 Percentile:37.22(Chemistry, Physical)Pressure-induced phase transition and polymerization of nitrogen-rich molecules are widely focused due to its extreme importance for the development of green high energy density materials. Here, we present a study of the phase transition and chemical reaction of 1H-tetrazole up to 100 GPa by using  Raman, IR, X-ray diffraction, neutron diffraction techniques and theoretical calculation. A phase transition above 2.6 GPa was identified and the high-pressure structure was determined with one molecule in a unit cell. The 1H-tetrazole polymerizes reversibly below 100 GPa, probably through a carbon-nitrogen bonding instead of nitrogen-nitrogen bonding. Our studies updated the structure model of the high pressure phase of 1H-tetrazole, and presented the possible intermolecular bonding route for the first time, which gives new insights to understand the phase transition and chemical reaction of nitrogen-rich compounds, and benefit for designing new high energy density materials.
Raman, IR, X-ray diffraction, neutron diffraction techniques and theoretical calculation. A phase transition above 2.6 GPa was identified and the high-pressure structure was determined with one molecule in a unit cell. The 1H-tetrazole polymerizes reversibly below 100 GPa, probably through a carbon-nitrogen bonding instead of nitrogen-nitrogen bonding. Our studies updated the structure model of the high pressure phase of 1H-tetrazole, and presented the possible intermolecular bonding route for the first time, which gives new insights to understand the phase transition and chemical reaction of nitrogen-rich compounds, and benefit for designing new high energy density materials.
Zhang, X. X.*; Knoop, D.*; Andr , H.*; Harjo, S.; Kawasaki, Takuro; Lutz, A.*; Lahres, M.*
, H.*; Harjo, S.; Kawasaki, Takuro; Lutz, A.*; Lahres, M.*
International Journal of Plasticity, 140, p.102972_1 - 102972_20, 2021/05
Times Cited Count:46 Percentile:94.90(Engineering, Mechanical)