Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 doped with neptunium
doped with neptuniumSato, Tsuyoshi*; Yamashita, Toshiyuki; Matsui, Tsuneo*
Journal of Nuclear Materials, 344(1-3), p.67 - 72, 2005/09
Times Cited Count:3 Percentile:23.58(Materials Science, Multidisciplinary)Phase relationships between NpO and CaTiO
and CaTiO or Ca(Ti, Al)O
or Ca(Ti, Al)O were investigated by X-ray diffraction (XRD) analysis, using specimens prepared at 1773 K in Ar-8%H
were investigated by X-ray diffraction (XRD) analysis, using specimens prepared at 1773 K in Ar-8%H . Single phase solid solutions were formed 0-7.5 mol%Np and 1-10 mol%Np for CaTiO
. Single phase solid solutions were formed 0-7.5 mol%Np and 1-10 mol%Np for CaTiO and Ca(Ti, Al)O
and Ca(Ti, Al)O , respectively. By substituting Al for Ti in CaTiO
, respectively. By substituting Al for Ti in CaTiO , Np solubility in Ca(Ti, Al)O
, Np solubility in Ca(Ti, Al)O increased. Solubility of Np was compared with those of U and Pu, and was discussed with oxidation states and ionic radii of dopants. Thermal expansions of (Ca,Np)TiO
increased. Solubility of Np was compared with those of U and Pu, and was discussed with oxidation states and ionic radii of dopants. Thermal expansions of (Ca,Np)TiO were measured from room temperature to 1273 K in Ar-8%H
were measured from room temperature to 1273 K in Ar-8%H using high-temperature XRD technique. These specimens showed nearly the same value of volumetric thermal expansion coefficients, suggesting that the incorporation of tetravalent Np into CaTiO
using high-temperature XRD technique. These specimens showed nearly the same value of volumetric thermal expansion coefficients, suggesting that the incorporation of tetravalent Np into CaTiO had practically no effect on stabilization of the crystal lattice. This finding was in a marked contrast to that of Pu doped CaTiO
had practically no effect on stabilization of the crystal lattice. This finding was in a marked contrast to that of Pu doped CaTiO , where pronounced stabilization in the crystal was observed by incorporating Pu into CaTiO
, where pronounced stabilization in the crystal was observed by incorporating Pu into CaTiO .
.
 Pu
Pu O
O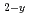 by EMF method
by EMF methodOtobe, Haruyoshi; Nakamura, Akio; Yamashita, Toshiyuki; Minato, Kazuo
Journal of Nuclear Materials, 344(1-3), p.219 - 222, 2005/09
Times Cited Count:3 Percentile:23.58(Materials Science, Multidisciplinary)Actinides-bearing zirconias are prominent candidate materials for various nuclear applications: targets for actinide transmutation, inert material fuels, radioactive waste forms, etc. In this study, the oxygen potential (g(O )) behavior of fluorite-type (F-type) Zr
)) behavior of fluorite-type (F-type) Zr Pu
Pu O
O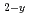 has been measured by EMF method using the zirconia oxygen sensor. It was found that the g(O
has been measured by EMF method using the zirconia oxygen sensor. It was found that the g(O ) values of F-type Zr
) values of F-type Zr Pu
Pu O
O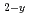 were about 150 kJ/mol higher than those of F-type PuO
were about 150 kJ/mol higher than those of F-type PuO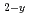 at the same oxygen-nonstoichiometric (O/M) values. The g(O
at the same oxygen-nonstoichiometric (O/M) values. The g(O ) values of F-type Zr
) values of F-type Zr Pu
Pu O
O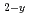 were 50 kJ/mol lower than those of pyrochlore Zr
were 50 kJ/mol lower than those of pyrochlore Zr Pu
Pu O
O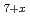 at the same O/M values. These results suggest that the g(O
at the same O/M values. These results suggest that the g(O ) behavior depends on the cation and anion ordering/disordering, in addition to the cation composition ratio (Zr/Pu).
) behavior depends on the cation and anion ordering/disordering, in addition to the cation composition ratio (Zr/Pu).
Kuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Journal of Nuclear Materials, 344(1-3), p.169 - 172, 2005/09
Times Cited Count:30 Percentile:86.30(Materials Science, Multidisciplinary)The knowledge of separation coefficients of actinides and rare-earth metals is important for developing pyrometallurgical process of spent nuclear fuel. Electrochemical experiments were carried out at 723-823 K to estimate separation coefficients in LiCl-KCl eutectic melt containing uranium and lanthanum trichlorides. Uranium and lanthanum separation coefficients is calcurated with the voltammetric peak potentials of U (III) and La (III), their concentration in the melt and kinetic parameters for U(III) discharge such as diffusion coefficients, and standard rate constants of charge transfer. The diffusion coefficients of U (III) were determined by some electrochemical measurements. The standard rate constants of charge transfer for electroreduction of uranium U(III) +3e =U were calculated by impedance spectroscopy method.
=U were calculated by impedance spectroscopy method.
Arai, Yasuo; Minato, Kazuo
Journal of Nuclear Materials, 344(1-3), p.180 - 185, 2005/09
Times Cited Count:24 Percentile:81.24(Materials Science, Multidisciplinary)no abstracts in English
Kobayashi, Tsuguyuki; Vavilov, S.; Myochin, Munetaka
Proceedings of 11th Symposium on Thermodynamics of Nuclear Materials (STNM-11), P. 91, 2004/00
The thermodunamic properties such as the Redox potential, diffusion coefficient, and oxidation rate constant of Pu are evaluated. These properties are used for the fieasibility study of the pyro-process.
Amamoto, Ippei; Terai, T.*; Sato, Koji
Proceedings of 11th Symposium on Thermodynamics of Nuclear Materials (STNM-11), P. 71, 2004/00
The application of the fluoride volatility process in the reprocessing of fuel from the fast breeder reactor is regarded as one of the economical methods. Plutonium hexafluoride (PuF6), however, reacting with fluorine (F2) and plutonium dioxide (PuO2) as the raw material, is in an unstable condition and tends to remain as a solid compound in the process after decomposing into plutonium tetrafluoride (PuF4). Suitable conditions should be established for the practical use of this process. One of them is to enhance the stability of PuF6. The behaviour of plutonium fluorination and relevant chemical reactions were investigated by referring to sundry literature and by thermodynamic calculation. It was then compared with recent data from laboratory scale experiments for this paper. Results from the theoretical analysis agreed with experimental observation that PuF6 could be formed stably under a high temperature condition (approx.1000 K) with over supply of figher concentration of F2.
Kato, Masato; Tamura, Tetsuya*; Aono, Shigenori
Proceedings of 11th Symposium on Thermodynamics of Nuclear Materials (STNM-11), P. 81, 2004/00
The oxygen potentials of (Pu U
U )O
)O were measured by thermogravimetric equilibrium measurement in the range of the temperature from 800 to 1350 in Ar/H
were measured by thermogravimetric equilibrium measurement in the range of the temperature from 800 to 1350 in Ar/H /H
/H O or He/H
O or He/H /H
/H O mixture gas flow. The experimental results of (Pu
O mixture gas flow. The experimental results of (Pu U
U )O
)O are in good agree with the other works. The relationship between the partial oxygen pressure (PO
are in good agree with the other works. The relationship between the partial oxygen pressure (PO ) and X in MO
) and X in MO was analized based on lattice defect theory. The oxygen potential of (Pu
was analized based on lattice defect theory. The oxygen potential of (Pu U
U )O
)O was modeled by lattice defect theory using the data of the literature and this work. The resulting equation well reproduces the oxygen potential-temperature data for (Pu
was modeled by lattice defect theory using the data of the literature and this work. The resulting equation well reproduces the oxygen potential-temperature data for (Pu U
U )O
)O .
.