Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Iijima, Kazuki; Tomura, Tsutomu*; Tobita, Minoru*; Suzuki, Yasuyuki*
Radiochimica Acta, 98(9-11), p.729 - 736, 2010/11
Times Cited Count:3 Percentile:23.88(Chemistry, Inorganic & Nuclear)Distribution behavior of Cs and Am in the synthetic groundwater-bentonite colloids-granite ternary system was investigated. Radionuclide sorbed onto the bentonite colloids is desorbed by addition of granite, indicating that the sorption of Cs and Am onto the bentonite colloids are reversible. The sorption model based on cation exchange and surface complexation reaction considering high edge site density for bentonite colloids is applicable to explain the sorption behavior of Am and Cs in the ternary system.
Tachi, Yukio; Nakazawa, Toshiyuki*; Ochs, M.*; Yotsuji, Kenji; Suyama, Tadahiro; Seida, Yoshimi; Yamada, Norikazu*; Yui, Mikazu
Radiochimica Acta, 98(9-11), p.711 - 718, 2010/11
Times Cited Count:25 Percentile:84.07(Chemistry, Inorganic & Nuclear)Diffusion and sorption of radionuclides in compacted bentonite are the key processes in the safe geological disposal. The effects of carbonate and salinity on Np(V) diffusion and sorption in compacted montmorillonite were investigated by experimental and modeling approaches. Effective diffusion coefficients (
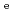 ) and distribution coefficients (
) and distribution coefficients (
 ) of Np in montmorillonite compacted to dry density of 800 kg/m
) of Np in montmorillonite compacted to dry density of 800 kg/m were measured under four conditions with different salinities (0.05/0.5 M NaCl) and carbonate concentrations (0/0.01 M NaHCO
were measured under four conditions with different salinities (0.05/0.5 M NaCl) and carbonate concentrations (0/0.01 M NaHCO ). The
). The 
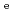 for carbonate-free conditions decreased as salinity increased, and those for carbonate conditions showed the opposite dependency. The
for carbonate-free conditions decreased as salinity increased, and those for carbonate conditions showed the opposite dependency. The 
 decreased by one order of magnitude under high carbonate condition. Diffusion and sorption behaviors were interpreted by coupling the thermodynamic aqueous speciation, the thermodynamic sorption model based on ion exchange and surface complexation, and the diffusion model based on electrical double layer theory in narrow pores. The mechanistic model could be useful in predicting the sorption and diffusion behavior of complex species in compacted systems.
decreased by one order of magnitude under high carbonate condition. Diffusion and sorption behaviors were interpreted by coupling the thermodynamic aqueous speciation, the thermodynamic sorption model based on ion exchange and surface complexation, and the diffusion model based on electrical double layer theory in narrow pores. The mechanistic model could be useful in predicting the sorption and diffusion behavior of complex species in compacted systems.
Seida, Yoshimi; Terashima, Motoki; Tachi, Yukio; Iijima, Kazuki; Nakazawa, Toshiyuki*; Yamada, Norikazu*; Yui, Mikazu
Radiochimica Acta, 98(9-11), p.703 - 709, 2010/11
Times Cited Count:3 Percentile:23.88(Chemistry, Inorganic & Nuclear)Diffusion and sorption behaviors of Eu in sedimentary rock were investigated in the presence of humic substance in the present study. Sedimentary rock obtained from Horonobe URL test site in Hokkaido, Japan, (accessible pore diameter is around several to several ten nanometer) was used. The Diffusion behaviors of Eu were examined based on reservoir depletion method coupled with analysis of inner distribution of the radioactive elements in the rock. Time sequence of concentration of each radioactive element as well as the humic substance in the reservoir was obtained as a function of concentration and molecular size of humic substance. Coexistence of humic substance reduced the depletion of Eu in the reservoir, indicating complexation between the radioactive elements and the humic substance. On the contrary, obvious decrease of humic substance in the reservoir was not observed in the system. This observation suggests that the radioactive elements became hard to diffuse into the sedimentary rock due to an increase of their size through complexation with the humic substance. The sorption of Eu was reduced with increase of the humic substance although the sorption of the humic substance was not influenced by the existence of Th or Eu. The diffusion and sorption of Eu were found to be reduced in the presence of humic substance.
Terashima, Motoki; Seida, Yoshimi; Iwatsuki, Teruki; Iijima, Kazuki; Yoshikawa, Hideki; Yui, Mikazu; Nagao, Seiya*
no journal, ,
Eu(III) binding abilities of dissolved humic substances (HSs), which were extracted from deep groundwater in the Horonobe Underground Research Center, Hokkaido, Japan, were investigated by fluorescence quenching technique. In the synthetic groundwater condition, the fluorescence quenching due to the binding with Eu(III) was not observed for the groundwater HSs, while that was observed for the Aldrich humic acid (HA). This result indicates that the binding ability of the groundwater HSs are different from that of Aldrich HA. Based on structural characteristics of the groundwater HSs, the differences in the binding ability are discussed.
Aoyagi, Noboru; Naganawa, Hirochika; Kimura, Takaumi
no journal, ,
Formation of poly-nuclear complexes of Ln(III) and An(III) ions are less feasible in an assumed conditions of underwater systems. They have been neglected because of low detection limits or difficulties in in-situ experimental analyses and lack of stockpile in knowledge. Conditions which promote to form poly-nuclear complexes are high total concentration of metal ions, moderate metal to ligand ratio in which part of ligands act as bridging donor, and high pH region in which complexation reaction compete with hydrolyses in the system involved. The existence of tri-nuclear Ln(III)-citrate is confirmed in stoichiometric amount with pH 9 -10. This large complex can be formed with several Ln(III) ions coexisted causing tri- and hetero-nuclear single complex. One fascinating behavior on the irradiation of steady or pulsed light is that they have strong intramolecular metal-to-metal energy transfer (MMET) in the presence of ligand because of meta-metal close separation. Even small amount of additive actinide ion such as high luminescent Cm(III) could still hold the inclusion within a large citrate complex. Time resolved spectroscopy on the addition of Cm(III) into the citrate system will be discussed in order to probe the local coordination geometry around Cm(III) which is more sensitive to chemical environment.
Ishida, Keisuke; Kimura, Takaumi; Saito, Takumi*; Tanaka, Satoru*
no journal, ,
no abstracts in English
Onuki, Toshihiko; Suzuki, Yoshinori; Nankawa, Takuya; Kozai, Naofumi; Francis, A. J.*
no journal, ,
We have examined the biological reduction of U(VI) complexed with organic acids of oxalic, tartaric, citric, and EDTA using S. putrefaciens. In biological system, the media containing lactic acid alone, concentration of the dissolved uranium decreased with time, and the precipitates were observed. The SEM and XANES analyses showed that the precipitates contained U, and its oxidation state was IV. In the other media containing oxalic, tartaric, citric, and EDTA, the concentration of dissolved uranium was almost the same as the initial one. The UV-vis spectra showed that dissolved U formed U(IV)-organic complexes in the medium containing oxalic, tartaric, citric acid or EDTA. These results suggest that the reduction of UVI by S. putrefaciens is affected by the organic acid present in the solution.
Sato, Haruo
no journal, ,
This paper presents a thermodynamic model which can calculate the interlayer space of Na-montmorillonite based on the relative partial molar Gibbs free energy of interlayer water in the Na-montmorillonite when contacted with a solution of an arbitrary salinity. Since the interlayer space of Na-montmorillonite cannot be directly calculated thermodynamically, here the average density of the Na-montmorillonite stacks and aggregates was calculated versus salinity. Based on the calculated results of Na-montmorillonite density versus salinity, interlayer space versus salinity was estimated. The calculated average density of montmorillonite stacks and aggregates increased with salinity, and particularly the change in density was significant in the low region of the initial montmorillonite density. The initial montmorillonite density of 0.5kg/dm increased to about 1.05kg/dm
increased to about 1.05kg/dm at the condition of 0.5M-NaCl, meanwhile the initial montmorillonite density of 1.0kg/dm
at the condition of 0.5M-NaCl, meanwhile the initial montmorillonite density of 1.0kg/dm increased to about 1.16kg/dm
increased to about 1.16kg/dm at the same salinity. The interlayer space estimated decreased with increasing salinity, and this trend was consistent with that of measured results.
at the same salinity. The interlayer space estimated decreased with increasing salinity, and this trend was consistent with that of measured results.
Sato, Haruo
no journal, ,
Swelling pressure versus Na-montmorillonite partial density was estimated for solutions of various salinities (pure water, NaCl solutions, artificial saline water, Horonobe groundwater) based on the thermodynamic data of the interlayer water of Na-montmorillonite and of water in the solutions of various salinities coming in contact with the montmorillonite and compared to data measured under the same salinities for various kinds and different silica sand contents of bentonites. The calculated swelling pressures decreased with increasing salinity. This is in good agreement with the fact that swelling pressure of bentonite generally decreases with increasing salinity. Since all measurements were carried out under the condition that soluble minerals remain being contained, all measured data are considered to contain the effect of the increase of the ionic strength of porewater by dissolution of the soluble minerals and the calculated values were within the range of the scattering in the measured data. By this model, swelling pressures for various Na-montmorillonite contents and silica sand contents of bentonites saturated with solutions of various salinities can be calculated as a function of the bentonites. Furthermore, an analysis for THMC coupled processes can be also carried out by considering those enthalpy and entropy data.
 O/D
O/D O systems
O systemsIshida, Keisuke; Saito, Takumi*; Aoyagi, Noboru; Kimura, Takaumi; Nagasaki, Shinya*; Tanaka, Satoru*
no journal, ,
Eu(III) surface complexes on kaolinite are investigated by TRLFS. It is as a model of clay mineral particles that can be released from engineered barrier of nuclear waste disposal or present as intrinsic aquatic or groundwater colloids. Kaolinite is a 1:1 aluminosilicate that has two reactive sites: Si-O site on the siloxane basal face and Al-O site on gibbsite-like edge face. It is probable that the outer-sphere complexes resulting from cation exchange at the Si-O site, while the inner-sphere complex is formed on the Al-O site. However, the reported lifetime of the inner-sphere complex showed the hydration number is 2.6, which does not agree with the structure of trivalent lanthanide on -Al O
O obtained from EXAFS. This discrepancy may suggest a change of character of coordinating H
obtained from EXAFS. This discrepancy may suggest a change of character of coordinating H O upon sorption or energy transfer from Eu(III) to the surface. The purpose of this study is to unveil these aspects and address the applicability of TRLFS. At the conference, we show the results of TRLFS measurements of Eu(III) on kaolinite in H
O upon sorption or energy transfer from Eu(III) to the surface. The purpose of this study is to unveil these aspects and address the applicability of TRLFS. At the conference, we show the results of TRLFS measurements of Eu(III) on kaolinite in H O/D
O/D O media and evaluate the roles of the surface as an energy accepter and/or an inhibitor affecting the quenching efficiency of H
O media and evaluate the roles of the surface as an energy accepter and/or an inhibitor affecting the quenching efficiency of H O coordinating to Eu(III) effects of the surface such as energy transfer and the quenching efficiency of H
O coordinating to Eu(III) effects of the surface such as energy transfer and the quenching efficiency of H O coordinating Eu(III).
O coordinating Eu(III).
Sao, Hirokazu*; Ishida, Keisuke; Saito, Takumi*; Aoyagi, Noboru; Nagasaki, Shinya*; Tanaka, Satoru*
no journal, ,
Structures of the Ln/An in solution and on mineral surfaces have been assessed by various spectroscopic techniques. In a relatively simple system containing a few species in solution this can be done unequivocally. However, on mineral surfaces at relatively high pH or in the presence of ligand molecules measured spectra are hard to interpret because of the co-existence of many species and/or the heterogeneity of the surfaces. In addition spectroscopic measurements involve several parameters, not only spectroscopic parameters like wavelength and delay time, but also chemical parameters like pH and concentrations of reactants. Therefore, the information on the number and relative amounts of different chemical species formed should be pursued in the entire multi-dimensional parameter space and one needs a systematic approach based on statistical argument for the purpose. The goal of this study is to construct a multivariable analysis method for TRLFS, which can extract the number of components, their pure spectra and the variation of their quantities from a series of spectra measured as a function of wavelength, time and chemical conditions. Uranyl sorption on gibbsite is studied by TRLFS as a test system for this purpose. It has been shown that the spectral shapes of uranyl on gibbsite varies as the delay time of the detector increases and as pH of the solution is changed, suggesting the presence of multiple sorption complexes of uranyl on gibbsite. The obtained TRLFS spectra are processed by the fixed-size window evolving factor analysis to extract the number of statistically meaningful components, followed by nonlinear least square regression for the deconvolution of temporally varying spectra.
Kitamura, Akira; Fujiwara, Kenso; Shibata, Masahiro; Tachi, Yukio; Doi, Reisuke; Yoshida, Yasushi*; Yamaguchi, Tetsuji; Yui, Mikazu
no journal, ,
A fundamental plan has been established to develop a thermodynamic database for performance assessment of geological disposal of high-level and TRU radioactive wastes. Number of selected elements is 24 (actinides, fission products and their daughters), which are of importance for the performance assessment of geological disposal. The fundamental plan is in principle based on the guidelines established by the Nuclear Energy Agency in the Organization for Economic Co-operation and Development (OECD/NEA). Additional unique guidelines have been established due to a requirement from the performance assessment to select tentative thermodynamic values obtained from chemical analogues and/or models for some elements of which thermodynamic values are insufficient. Status on selection of thermodynamic data of cobalt, nickel and palladium has been introduced as well as the fundamental plan.
Ochs, M.*; Kunze, S.*; Dahinden, M.*; Tachi, Yukio; Yui, Mikazu
no journal, ,
To predict retention of radionuclides in engineered barriers for radioactive waste disposal, a thermodynamic sorption model for key radionuclide, Ni(II), Am(III), Th(IV), Np(V), U(VI) and Se(IV) on clay minerals and bentonite was developed as a function of chemical conditions. The basic premises are to keep model characteristics relatively simple and to consistently use the same basic model design and parameters. For this objectives, a simple 1-site surface complexation/diffuse layer model in combinations with 1-site ion exchange was selected. Basic parameters were evaluated from surface titration data obtained at 0.01/0.1/0.5 molar background electrolyte. The resulting basic model was then parameterized on the basis of selected published original sorption data of radionuclides. The parameterized models were further evaluated against additional independent datasets. The developed sorption models and results of their application to various radionuclide sorption data are presented.