Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

Suzuki, Kiichi; Murakami, Tatsutoshi; Hirooka, Shun; Nelson, A.*; McClellan, K.*; Kato, Masato
no journal, ,
The relationship between equilibrium oxygen partial pressure, 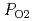 , and deviation from stoichiometric composition, x, was evaluated using a Kroger-Vink diagram. It was found that x was proportional to
, and deviation from stoichiometric composition, x, was evaluated using a Kroger-Vink diagram. It was found that x was proportional to 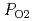
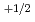 and
and 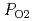
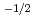 near stoichiometry for all compositions. This suggests that intrinsic ionization is dominant near stoichiometry. Also, in the reducing region, x was proportional to
near stoichiometry for all compositions. This suggests that intrinsic ionization is dominant near stoichiometry. Also, in the reducing region, x was proportional to 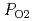
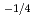 for 20 and 30% of Ce, and was proportional to
for 20 and 30% of Ce, and was proportional to 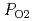
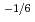 for 5% of Ce. Defect reactions and defect concentrations were evaluated by the relationship between x and
for 5% of Ce. Defect reactions and defect concentrations were evaluated by the relationship between x and 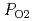
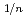 . Then, the oxygen potential expression was derived using the defect concentrations as functions of O/M ratio, temperature and Ce content. The calculated values reproduced experimental values well.
. Then, the oxygen potential expression was derived using the defect concentrations as functions of O/M ratio, temperature and Ce content. The calculated values reproduced experimental values well.
Hirooka, Shun; Nelson, A.*; White, J.*; Kato, Masato; McClellan, K.*
no journal, ,
Thermal diffusivity of (U Ce
Ce )O
)O and (U
and (U Ce
Ce )O
)O was measured by laser flash method as a function of oxygen-to-metal ratio over the temperature range of 373 K to 1473 K and the thermal conductivity was evaluated. Only stoichiometric data of thermal conductivity of (U,Ce)O
was measured by laser flash method as a function of oxygen-to-metal ratio over the temperature range of 373 K to 1473 K and the thermal conductivity was evaluated. Only stoichiometric data of thermal conductivity of (U,Ce)O was reported and that was comparable with thermal conductivity obtained in this work. Thermal conductivity was highest at stoichiometry, and was not perfectly symmetric with respect to the stoichiometry but slightly lower for hyper-stoichiometric compositions. The thermal conductivity of (U,Ce)O
was reported and that was comparable with thermal conductivity obtained in this work. Thermal conductivity was highest at stoichiometry, and was not perfectly symmetric with respect to the stoichiometry but slightly lower for hyper-stoichiometric compositions. The thermal conductivity of (U,Ce)O showed the trend with temperature to be comparable to that of (U,Pu)O
showed the trend with temperature to be comparable to that of (U,Pu)O . However, the thermal resistivity did not follow the linear function of temperature, A+BT, which is an unique characteristics in (U,Ce)O
. However, the thermal resistivity did not follow the linear function of temperature, A+BT, which is an unique characteristics in (U,Ce)O .
.