Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kitawaki, Shinichi; Shinozaki, Tadahiro; Fukushima, Mineo; Usami, Tsuyoshi*; Yahagi, Noboru*; Kurata, Masaki*
Nuclear Technology, 162(2), p.118 - 123, 2008/05
Times Cited Count:18 Percentile:72.94(Nuclear Science & Technology)A series test of pyro-process was carried out to recover U-Pu alloy from MONJU MOX pellets. In the Li-reduction step, the reduction behavior of MOX was similar to that of UO . In the electrorefining step, the separation factor between U and Pu was 5.7 for the combination of reduced MOX anode and liquid cadmium cathode, which is almost comparable to the value in the previous studies. For the material balance, approximately 98% of U and 103% of Pu were detected in the electrodes or molten salt after the electrolysis with respect to the initial amounts in the anode or molten salt. Considering the analytical error of ICP-AES, these values are reasonable. The remained amount of U in the anode was slightly larger than that of Pu due to the re-oxidation. The U-Pu alloy ingot was successfully formed by distillation of Cd.
. In the electrorefining step, the separation factor between U and Pu was 5.7 for the combination of reduced MOX anode and liquid cadmium cathode, which is almost comparable to the value in the previous studies. For the material balance, approximately 98% of U and 103% of Pu were detected in the electrodes or molten salt after the electrolysis with respect to the initial amounts in the anode or molten salt. Considering the analytical error of ICP-AES, these values are reasonable. The remained amount of U in the anode was slightly larger than that of Pu due to the re-oxidation. The U-Pu alloy ingot was successfully formed by distillation of Cd.
Minato, Kazuo; Arai, Yasuo
no journal, ,
no abstracts in English
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo; Haire, R. G.*
no journal, ,
The electrochemical behavior of Am at a liquid Cd cathode and the alloying behavior of Am with Cd were analyzed. In the cyclic voltammograms obtained with Mo electrode, the redox reactions of Am(III)/Am(II) and Am(II)/Am were observed, while peaks assigned to Am(III)/Am (in Cd) were observed with liquid Cd electrode. The observed electric potentials for the peaks with liquid Cd electrode were more positive than those with Mo electrode. The difference in the potentials  E(Am-Cd) was 0.39V, which is similar to
E(Am-Cd) was 0.39V, which is similar to  E(Pu-Cd) and much larger than
E(Pu-Cd) and much larger than  E(U-Cd) and
E(U-Cd) and  E(Np-Cd). It is reported that
E(Np-Cd). It is reported that  E(An-Cd) (An=U, Np, Pu) corresponds to
E(An-Cd) (An=U, Np, Pu) corresponds to  G
G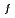 of An-Cd compound formed at Cd electrode (UCd
of An-Cd compound formed at Cd electrode (UCd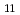 , NpCd
, NpCd , PuCd
, PuCd ). On the other hand, to identify the stable chemical form on Am-Cd phase diagram, Am-Cd intermetallic compounds were prepared by heating
). On the other hand, to identify the stable chemical form on Am-Cd phase diagram, Am-Cd intermetallic compounds were prepared by heating 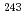 Am metal with liquid Cd. The product prepared by heating at 723K was identified as cubic AmCd
Am metal with liquid Cd. The product prepared by heating at 723K was identified as cubic AmCd with a
with a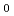 =1.559
=1.559  0.002 nm by powder X-ray diffraction. AmCd
0.002 nm by powder X-ray diffraction. AmCd is considered to be formed at Cd electrode on the electrochemical experiments because it is the stable product at 723K.
is considered to be formed at Cd electrode on the electrochemical experiments because it is the stable product at 723K.