Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Honjo, Eijiro; Tamada, Taro; Adachi, Motoyasu; Kuroki, Ryota; Meher, A.*; Blaber, M.*
Journal of Synchrotron Radiation, 15(3), p.285 - 287, 2008/05
Times Cited Count:5 Percentile:29.06(Instruments & Instrumentation)We attempt to improve a crystal contact of human acidic fibroblast growth factor (haFGF1) to control the crystal growth because haFGF crystallizes only as a thin-plate form. X-ray crystal analysis of haFGF showed that side chain Glu81, located at a crystal contact between haFGF molecules related by crystallographic symmetry, were in close proximity, suggesting that charge-repulsion may disrupt suitable crystal-packing interaction. To investigate whether the Glu residue affects crystal packing, we constructed haFGF mutants. Although crystals of Ala, Val and Leu mutants were grown as a thin-plate form by the same precipitant (formate) as wild type, crystals of Ser and Thr mutants were grown as a more bulky form. X-ray structural analysis of Ser and Thr mutants determined at 1.5 resolution revealed that hydroxyl groups of Ser and Thr were linked by hydrogen bonds mediated by formate used as a precipitant.
resolution revealed that hydroxyl groups of Ser and Thr were linked by hydrogen bonds mediated by formate used as a precipitant.
Kurihara, Kazuo; Tamada, Taro; Ohara, Takashi; Kuroki, Ryota
no journal, ,
Ohara, Takashi; Umino, Hisao; Tamada, Taro; Kuroki, Ryota
no journal, ,
no abstracts in English
Arai, Shigeki; Tamada, Taro; Maeda, Yoshitake*; Kuroki, Ryota
no journal, ,
The mouse antibody TN1 recognizes the human thrombopoietin (hTPO) that primarily stimulates megakaryocytopoiesis and platelet production. In order to clarify the mechanism of the neutralizing activity of the TN1 antibody, the crystal structure of TN1-Fab was determined to 2.0 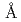 resolution and was compared with that of TN1-Fab / hTPO complex (PDB id 1V7M and 1V7N). The relative angle of the variable- and constant-regions of the hTPO unbound form of TN1-Fab was slightly shifted from those of TN1-Fab / hTPO complex (rms deviation = 2.4
resolution and was compared with that of TN1-Fab / hTPO complex (PDB id 1V7M and 1V7N). The relative angle of the variable- and constant-regions of the hTPO unbound form of TN1-Fab was slightly shifted from those of TN1-Fab / hTPO complex (rms deviation = 2.4 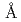 for all C
for all C atoms of Fab). On the other hand, only the slight shift of the side chain of CDR was observed (rmsd
atoms of Fab). On the other hand, only the slight shift of the side chain of CDR was observed (rmsd  1.5
1.5 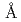 for atoms of side chain of each CDR) upon recognition of the epitope of hTPO. This structural shift of side chain of paratope did not accompany the
for atoms of side chain of each CDR) upon recognition of the epitope of hTPO. This structural shift of side chain of paratope did not accompany the 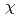 -angle rotation, but the parallel shift. These results indicate that the CDR of TN1-Fab need not accompany the large conformational changes of upon TPO recognition.
-angle rotation, but the parallel shift. These results indicate that the CDR of TN1-Fab need not accompany the large conformational changes of upon TPO recognition.
Adachi, Motoyasu; Tamada, Taro; Kurihara, Kazuo; Oga, Takushi*; Kuramitsu, Seiki*; Kuroki, Ryota
no journal, ,
ADP ribose pyrophosphatase (ADPRase) catalyzes the hydrolysis of ADP ribose to ribose 5' phosphate and AMP. In order to clarify the catalytic mechanism of ADPRase including ionization state of catalytic residues and role of water molecules on its catalytic function, recombinant ADPRase was expressed in  and a large crystal was prepared by macro seeding. After the two months repeat of the macro seeding, a large crystal with the dimensions of 3.2
and a large crystal was prepared by macro seeding. After the two months repeat of the macro seeding, a large crystal with the dimensions of 3.2 2.8
2.8 1.5 (13 mm
1.5 (13 mm ) was grown in the sodium acetate buffer (pH 5.3) containing 20% glycerol, 0.2M ammonium sulfate, and 18% PEG4000 at 20
) was grown in the sodium acetate buffer (pH 5.3) containing 20% glycerol, 0.2M ammonium sulfate, and 18% PEG4000 at 20 C. Then, the crystal was soaked into the reservoir solution prepared using deuterated reagents for 20 days, and was mounted to a BIX-3 neutron diffractometer installed at the research reactor JRR-3 in JAEA. As a result of the preliminary experiments, neutron diffraction spots up to 2.1
C. Then, the crystal was soaked into the reservoir solution prepared using deuterated reagents for 20 days, and was mounted to a BIX-3 neutron diffractometer installed at the research reactor JRR-3 in JAEA. As a result of the preliminary experiments, neutron diffraction spots up to 2.1  resolution were observed in the still images taken by 240 minutes exposure.
resolution were observed in the still images taken by 240 minutes exposure.
Tamada, Taro; Honjo, Eijiro; Maeda, Yoshitake*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
no journal, ,
We have succeeded in crystallization of 2:2 complex between human granulocyte colony-stimulating factor (hGCSF) and the Ig-like and CRH domains of human GCSF-R (hGCSF-R) and determined its tertiary structure by X-ray crystallography at 2.8  resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6
resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6  -receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
-receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
Yamaguchi, Shigeo*; Kamikubo, Hironari*; Kurihara, Kazuo; Shimizu, Tetsuya*; Yamazaki, Yoichi*; Kuroki, Ryota; Niimura, Nobuo*; Kataoka, Mikio*
no journal, ,
Shoyama, Yoshinari; Tamada, Taro; Takeuchi, Ayako*; Taura, Futoshi*; Shoyama, Yukihiro*; Kuroki, Ryota; Morimoto, Satoshi*
no journal, ,
Chatake, Toshiyuki*; Kurihara, Kazuo; Kuroki, Ryota; Morimoto, Yukio*
no journal, ,