Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Mo adsorption and
Mo adsorption and  Tc elution from zirconium-based material in
Tc elution from zirconium-based material in  Mo/
Mo/ Tc generator column using neutron-irradiated natural molybdenum
Tc generator column using neutron-irradiated natural molybdenumAwaludin, R.*; Gunawan, A. H.*; Lubis, H.*; Sriyono*; Herlina*; Mutalib, A.*; Kimura, Akihiro; Tsuchiya, Kunihiko; Tanase, Masakazu*; Ishihara, Masahiro
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1481 - 1483, 2015/02
Times Cited Count:9 Percentile:56.63(Chemistry, Analytical)In this study, the  Mo adsorption and
Mo adsorption and  Tc elution mechanism were investigated using SEM-EDS to analyze the elemental composition of the material surfaces before Mo adsorption, after Mo adsorption and after
Tc elution mechanism were investigated using SEM-EDS to analyze the elemental composition of the material surfaces before Mo adsorption, after Mo adsorption and after  Tc elution using saline solution. The results were compared with the value of adsorption capacity of the material to irradiated natural Mo and elution yield of
Tc elution using saline solution. The results were compared with the value of adsorption capacity of the material to irradiated natural Mo and elution yield of  Tc. From the changes of elemental composition in the surface, it was found that molybdate ions were adsorbed into the adsorbent by ion exchange with Cl
Tc. From the changes of elemental composition in the surface, it was found that molybdate ions were adsorbed into the adsorbent by ion exchange with Cl ion in the material. On the other hand, it was also revealed that
ion in the material. On the other hand, it was also revealed that  Tc can be eluted from the material column in TcO
Tc can be eluted from the material column in TcO
 since oxidizing agent was needed in the elution process.
since oxidizing agent was needed in the elution process.
Shimada, Taro; Tanaka, Tadao
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1345 - 1349, 2015/02
Times Cited Count:11 Percentile:63.96(Chemistry, Analytical)In order to understand the production and dispersion behavior of radioactive aerosols during dismantling of nuclear facilities, plasma arc cutting experiments were conducted. Particle size distribution of the aerosols was obtained by sampling air into ELPI which could classify particles into 12 stages of 50% cutoff aerodynamic diameter ranging from 0.007 to 9.9  m.
m.  Co specific radioactivity of diameter 0.05
Co specific radioactivity of diameter 0.05  m during cutting of surface contaminated piping indicated the maximum value of approximately 2.7E+4 Bq/g which was fifty times as much as the average value of all of aerosols. That of 9.9
m during cutting of surface contaminated piping indicated the maximum value of approximately 2.7E+4 Bq/g which was fifty times as much as the average value of all of aerosols. That of 9.9 m was approximately 100 Bq/g which was the eighth part of the average value. Compared with those for the activated piping, the difference of specific radioactivity between maximum and minimum values were larger in contaminated piping. It is considered that contaminants on the piping were directly melted and vaporized by plasma arc and then condensed into smaller particles.
m was approximately 100 Bq/g which was the eighth part of the average value. Compared with those for the activated piping, the difference of specific radioactivity between maximum and minimum values were larger in contaminated piping. It is considered that contaminants on the piping were directly melted and vaporized by plasma arc and then condensed into smaller particles.
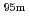 Tc for Compton camera imaging
Tc for Compton camera imagingHatsukawa, Yuichi; Hashimoto, Kazuyuki; Tsukada, Kazuaki; Sato, Tetsuya; Asai, Masato; Toyoshima, Atsushi; Nagai, Yasuki; Tanimori, Toru*; Sonoda, Shinya*; Kabuki, Shigeto*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1283 - 1285, 2015/02
Times Cited Count:2 Percentile:16.17(Chemistry, Analytical)Technetium-99m ( Tc) is used in radioactive medical diagonostic tests, for example as a radioactive tracer that medical equipment can detect in the human body. It is well suited to the role because it emits readily detectable 141 keV
Tc) is used in radioactive medical diagonostic tests, for example as a radioactive tracer that medical equipment can detect in the human body. It is well suited to the role because it emits readily detectable 141 keV  rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). There are at least 31 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood, and tumors. Recent years, with the develop-ment of the Compton camera which can realize high position resolution, technetium isotopes emitting high energy
rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). There are at least 31 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood, and tumors. Recent years, with the develop-ment of the Compton camera which can realize high position resolution, technetium isotopes emitting high energy  -rays are required. In this study, technetium-95m which emits some
-rays are required. In this study, technetium-95m which emits some  rays around 800 keV was produced by the
rays around 800 keV was produced by the 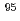 Mo(p,n)
Mo(p,n)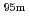 Tc reaction.
Tc reaction.
Watanabe, Satoshi; Hashimoto, Kazuyuki; Ishioka, Noriko
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1519 - 1521, 2015/02
Times Cited Count:13 Percentile:69.58(Chemistry, Analytical)As basic studies of bifunctional chelating agent for 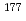 Lu-labeled antibodies,
Lu-labeled antibodies,  Lu complexation of DOTA and DTPA was investigated by the addition of competing metals, Ca(II), Fe(II) and Zn(II). From comparison of competing metals, the inhibition by competing metals on the
Lu complexation of DOTA and DTPA was investigated by the addition of competing metals, Ca(II), Fe(II) and Zn(II). From comparison of competing metals, the inhibition by competing metals on the  Lu complexation was in the order of Ca(II)
Lu complexation was in the order of Ca(II)  Fe(II)
Fe(II)  Zn(II) and Ca(II)
Zn(II) and Ca(II)  Zn(II)
Zn(II)  Fe(II) as DOTA and DTPA, respectively. For comparison between DOTA and DTPA, the susceptibility to the inhibition on
Fe(II) as DOTA and DTPA, respectively. For comparison between DOTA and DTPA, the susceptibility to the inhibition on  Lu complexation by all the three competing metals was DTPA
Lu complexation by all the three competing metals was DTPA  DOTA. Therefore, it was found that DTPA is advantageous for
DOTA. Therefore, it was found that DTPA is advantageous for  Lu complexation compared with DOTA in the presence of Ca(II), Fe(II) and Zn(II), and that the elimination of Fe from
Lu complexation compared with DOTA in the presence of Ca(II), Fe(II) and Zn(II), and that the elimination of Fe from 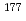 Lu solution is especially effective because the
Lu solution is especially effective because the 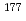 Lu complexation of DTPA is highly inhibited by Fe(II).
Lu complexation of DTPA is highly inhibited by Fe(II).
Matsunaga, Takeshi; Nakanishi, Takahiro; Atarashi-Andoh, Mariko; Takeuchi, Erina; Tsuzuki, Katsunori; Nishimura, Shusaku; Koarashi, Jun; Otosaka, Shigeyoshi; Sato, Tsutomu*; Nagao, Seiya*
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1291 - 1295, 2015/02
Times Cited Count:3 Percentile:23.54(Chemistry, Analytical)An innovative, yet simple method for the passive collection of radioactive materials in river water has been developed and validated. The method employes large filter vessels, containing multiple cartridge filters. River water is led to the system naturally using a drop of the riverbed by hose from upstream. This method makes long-term, unmanned monitoring possible. In addition to regular radioactivity analyses, this method provides an opportunity for the characterization of suspended materials based on its ample collection quantities (more than several tens of grams). This method may also be applicable to sediment-bound chemicals.
 -rays
-raysArisaka, Makoto; Watanabe, Masayuki; Ishizaki, Manabu*; Kurihara, Masato*; Chen, R.*; Tanaka, Hisashi*
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1543 - 1547, 2015/02
Times Cited Count:16 Percentile:74.22(Chemistry, Analytical)The influence of irradiation with  -rays to metal hexacyanoferrate (MHCF: M = Fe, Cu or Ni), which is known as an adsorbent for selective adsorption of cesium (Cs) ion in solution, on Cs adsorption ability and stability was investigated in HNO
-rays to metal hexacyanoferrate (MHCF: M = Fe, Cu or Ni), which is known as an adsorbent for selective adsorption of cesium (Cs) ion in solution, on Cs adsorption ability and stability was investigated in HNO solutions. Under the adsorbed dose conditions (50-300 kGy), it was found that the MHCF is fully stable although the radiolytic decomposition of MHCF was slightly observed with an increase of the total adsorbed dose, which was confirmed by an increment of Fe, Cu or Ni concentration in HNO
solutions. Under the adsorbed dose conditions (50-300 kGy), it was found that the MHCF is fully stable although the radiolytic decomposition of MHCF was slightly observed with an increase of the total adsorbed dose, which was confirmed by an increment of Fe, Cu or Ni concentration in HNO solution after the irradiation. The weight percent of the metal in the solution to initial weight of MHCF was less than unity. Moreover, no change in composition of carbon, hydrogen and nitrogen in MHCF was observed. On the other hand, the distribution coefficients of Cs to the irradiated MHCF were independent of the total adsorbed dose. This indicates that the Cs adsorption ability was maintained under
solution after the irradiation. The weight percent of the metal in the solution to initial weight of MHCF was less than unity. Moreover, no change in composition of carbon, hydrogen and nitrogen in MHCF was observed. On the other hand, the distribution coefficients of Cs to the irradiated MHCF were independent of the total adsorbed dose. This indicates that the Cs adsorption ability was maintained under  -ray irradiation.
-ray irradiation.
 -radiation effect on solvent extraction of minor actinide
-radiation effect on solvent extraction of minor actinideSugo, Yumi; Sasaki, Yuji; Taguchi, Mitsumasa; Ishioka, Noriko
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1381 - 1384, 2015/02
Times Cited Count:5 Percentile:36.53(Chemistry, Analytical) gas-jet system coupled to a surface-ionization type ion-source in JAEA-ISOL; Towards determination of the first ionization potential of Lr (Z = 103)
gas-jet system coupled to a surface-ionization type ion-source in JAEA-ISOL; Towards determination of the first ionization potential of Lr (Z = 103)Sato, Tetsuya; Asai, Masato; Sato, Nozomi; Tsukada, Kazuaki; Toyoshima, Atsushi; Oe, Kazuhiro*; Miyashita, Sunao*; Kaneya, Yusuke; Osa, Akihiko; Sch del, M.; et al.
del, M.; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1253 - 1257, 2015/02
Times Cited Count:9 Percentile:56.63(Chemistry, Analytical)We have developed a surface-ionization ion-source coupled to the He/CdI gas-jet transport system for the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator for experimental determination of the first ionization potential of lawrencium (Lr,
gas-jet transport system for the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator for experimental determination of the first ionization potential of lawrencium (Lr, 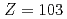 ). We performed to ionize a short-lived Lr isotope and various lanthanide isotopes. We successfully observed mass-separated ions of
). We performed to ionize a short-lived Lr isotope and various lanthanide isotopes. We successfully observed mass-separated ions of  Lr by using our present system at the first time. The first ionization potential of Lr was evaluated based on a correlation between of effective ionization potential and ionization efficiency of short-lived lanthanide isotopes in our system.
Lr by using our present system at the first time. The first ionization potential of Lr was evaluated based on a correlation between of effective ionization potential and ionization efficiency of short-lived lanthanide isotopes in our system.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1331 - 1334, 2015/02
Times Cited Count:1 Percentile:8.83(Chemistry, Analytical)Electrochemical behavior of Am in NaCl-2CsCl melt at 823 K was investigated by transient electrochemical techniques such as cyclic voltammetry and differential pulse voltammetry. The results show that Am(III) ion is reduced to Am metal by a two-step mechanism via Am(II) ion. Formal standard potential of Am(III)/Am(II) and that of Am(II)/Am(0) redox couples have been determined to be -2.73 and -2.97 V vs Cl /Cl
/Cl , respectively.
, respectively.
 Rn-
Rn- At generator
At generatorMaeda, Eita*; Yokoyama, Akihiko*; Taniguchi, Takumi*; Washiyama, Koshin*; Nishinaka, Ichiro
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1465 - 1468, 2015/02
Times Cited Count:12 Percentile:67.19(Chemistry, Analytical)The  At isotope has gathered attention as a promising
At isotope has gathered attention as a promising  -emitter for radionuclide therapy. We report the dependence of the distribution ratio of astatine on the concentration of HCl, and on the polarity of the organic solvent. The results will be useful for development of the
-emitter for radionuclide therapy. We report the dependence of the distribution ratio of astatine on the concentration of HCl, and on the polarity of the organic solvent. The results will be useful for development of the  Rn-
Rn- At generator.
At generator.
Aoyagi, Noboru; Watanabe, Masayuki; Kirishima, Akira*; Sato, Nobuaki*; Kimura, Takaumi
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1095 - 1098, 2015/02
Times Cited Count:6 Percentile:42.50(Chemistry, Analytical)Nagaoka, Mika; Yokoyama, Hiroya; Fujita, Hiroki; Nakano, Masanao; Watanabe, Hitoshi; Sumiya, Shuichi
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1305 - 1308, 2015/02
Times Cited Count:18 Percentile:78.00(Chemistry, Analytical)Various kinds of radionuclides were released into the atmosphere and the sea from the Fukushima Daiichi Nuclear Power Plant on Tokyo Electric Power Co. (TEPCO) by the accident and then reached around our laboratories. Therefore the accident influence on our environment was investigated to measure the concentrations of cesium-134 ( Cs), cesium-137
Cs), cesium-137  Cs, strontium-90 (
Cs, strontium-90 ( Sr) and plutonium isotopes in seabed sediments. The values for
Sr) and plutonium isotopes in seabed sediments. The values for  Cs ranged from 6.1 to 300 Bq/kg (dry wt) and the ratio of
Cs ranged from 6.1 to 300 Bq/kg (dry wt) and the ratio of  Cs /
Cs / Cs ranged from 0.48 to 0.77. The highest point of
Cs ranged from 0.48 to 0.77. The highest point of  Cs concentration was observed at the northernmost station near Kitaibaraki City and the concentration was similar to report by MEXT.
Cs concentration was observed at the northernmost station near Kitaibaraki City and the concentration was similar to report by MEXT.
 I in the Accumulated radioactive water and processing water of the Fukushima Daiichi Nuclear Power Plant
I in the Accumulated radioactive water and processing water of the Fukushima Daiichi Nuclear Power PlantShimada, Asako; Sakatani, Keiichi; Kameo, Yutaka; Takahashi, Kuniaki
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1137 - 1140, 2015/02
Times Cited Count:6 Percentile:42.50(Chemistry, Analytical)Accumulated radioactive water and processing water were sampled from back and forth of the Accumulated radioactive water processing facility (ARWPF) at Fukushima Daiichi Nuclear Power Plant (FDNPP) to estimate the radioactivity of the secondary waste such as zeolite and sludge adsorbed radioactive material. Separation method of I from the radioactive materials using solid phase extractant, Anion-SR, was developed, and the concentration of
from the radioactive materials using solid phase extractant, Anion-SR, was developed, and the concentration of  I in the accumulated water and processing water was determined by Inductively Coupled Plasma Mass Spectrometry including dynamic reaction cell (DRC-ICP-MS).
I in the accumulated water and processing water was determined by Inductively Coupled Plasma Mass Spectrometry including dynamic reaction cell (DRC-ICP-MS).
Rudolph, D.*; Forsberg, U.*; Golubev, P.*; Sarmiento, L. G.*; Yakushev, A.*; Andersson, L.-L.*; Di Nitto, A.*; D llmann, Ch. E.*; Gates, J. M.*; Gregorich, K. E.*; et al.
llmann, Ch. E.*; Gates, J. M.*; Gregorich, K. E.*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1185 - 1190, 2015/02
Times Cited Count:8 Percentile:52.35(Chemistry, Analytical)Thirty correlated  -decay chains of element 115 were observed, which were consistent with previous observations interpreted as the decay chain of
-decay chains of element 115 were observed, which were consistent with previous observations interpreted as the decay chain of 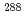 115. GEANT4 Monte-Carlo simulations were performed to reproduce high-resolution
115. GEANT4 Monte-Carlo simulations were performed to reproduce high-resolution  -photon coincidence results, which allows one to propose Q
-photon coincidence results, which allows one to propose Q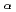 values and excitation schemes of the superheavy nuclei with unprecedented precision.
values and excitation schemes of the superheavy nuclei with unprecedented precision.
Kimura, Takaumi
no journal, ,
no abstracts in English
Kaneya, Yusuke; Sato, Tetsuya; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Miyashita, Sunao*; Nagame, Yuichiro; Sch del, M.; Sato, Nozomi; Oe, Kazuhiro*; et al.
del, M.; Sato, Nozomi; Oe, Kazuhiro*; et al.
no journal, ,
We have developed a surface ionization ion-source as part of the JAEA-ISOL that is coupled to a He/CdI gas-jet transport system to determine the first ionization potential (IP) of heavy actinides. Separation efficiencies of various short-lived lanthanide isotopes as homologs of actinides that were produced in nuclear reactions were measured with the present system. Obtained results demonstrate that the developed ion-source would be applicable to a measurement of the IP of heavy actinides.
gas-jet transport system to determine the first ionization potential (IP) of heavy actinides. Separation efficiencies of various short-lived lanthanide isotopes as homologs of actinides that were produced in nuclear reactions were measured with the present system. Obtained results demonstrate that the developed ion-source would be applicable to a measurement of the IP of heavy actinides.
Toyoshima, Atsushi; Miyashita, Sunao*; Asai, Masato; Sato, Tetsuya; Kaneya, Yusuke; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Nagame, Yuichiro; Sch del, M.; Lerum, H. V.*; et al.
del, M.; Lerum, H. V.*; et al.
no journal, ,
To carry out a continuous reduction experiment of Sg with the low production rates and the short half-life, we are developing a new chemistry assembly consisting of a membrane degasser (MDG), a flow electrolytic column (FEC), the continuous liquid-liquid extraction apparatus, and the liquid scintillation counting system (SISAK). Recently, we have begun preparatory studies with Mo and W isotopes. Aqueous solution dissolving Mo and W was successfully separated from a carrier gas. Redox couples of Mo(VI)/Mo(V) and W(VI)/W(V) in HCl have been characterized for their macro amounts. Extraction behavior of Mo(VI) and W(VI) between toluene containing hinokitiol (HT) and HCl was successfully observed by a batch method. On-line extractions of short-lived Mo and W were also carried out using SISAK and MDG. In the symposium, our present status of the preparation with Mo and W will be presented.
 Cu-labeled MARSGL peptide as an imaging probe for HER2/neu overexpressing tumors
Cu-labeled MARSGL peptide as an imaging probe for HER2/neu overexpressing tumorsSugo, Yumi; Sasaki, Ichiro; Watanabe, Shigeki; Ohshima, Yasuhiro; Ishioka, Noriko
no journal, ,
Minato, Kazuo
no journal, ,
Towards the decommissioning of Fukushima Daiichi Nuclear Power Plants, science-based research and development is important and useful, as well as technology and engineering development. Research and development activities based on radiation chemistry, radiochemistry, thermodynamics, etc., have contributed to safe and efficient decommissioning of the plants. These activities performed by the Japan Atomic Energy Agency are presented.
Takahatake, Yoko; Nakamura, Masahiro; Shibata, Atsuhiro; Koma, Yoshikazu; Nomura, Kazunori; Nakajima, Yasuo
no journal, ,