Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Honda, Mitsunori; Shimoyama, Iwao; Baba, Yuji; Suzuki, Shinichi; Okamoto, Yoshihiro; Yaita, Tsuyoshi
e-Journal of Surface Science and Nanotechnology (Internet), 14, p.35 - 38, 2016/02
Matsumura, Daiju; Nishihata, Yasuo; Okajima, Yuka*
e-Journal of Surface Science and Nanotechnology (Internet), 14, p.48 - 52, 2016/00
Baba, Yuji; Shimoyama, Iwao; Hirao, Norie; Izumi, Toshinori
e-Journal of Surface Science and Nanotechnology (Internet), 13, p.417 - 421, 2015/09
Times Cited Count:1The interaction between alkali metals and oxides has attracted much attention as heterogeneous catalysis, chemical reaction promoters, and high intensity electron emitter. Also the interaction of cesium and oxides has become important subject, because radioactive cesium trapped in minerals such as clay and soil may cause health risks. In the present study, we analyzed chemical states of ultra-trace amount of cesium on oxide surfaces by total reflection X-ray photoelectron spectroscopy (TR-XPS) exited by synchrotron radiation. For the adsorbed cesium thicker than 0.01 layer, cesium is weakly bound with oxide through Van-der-Waals force. On the other hand, for ultra-thin layer about 0.002 layer, the chemical bond changes to covalent bond. It is suggested that this change in the chemical bonding state is one of the reasons why radioactive cesium is hard to be released from minerals.
 Ce
Ce O
O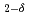 for electrolyte by Rietveld/ maximum entropy method
for electrolyte by Rietveld/ maximum entropy methodTaguchi, Tomitsugu; Igawa, Naoki; Birumachi, Atsushi; Asaoka, Hidehito; Miwa, Shuhei; Osaka, Masahiko
e-Journal of Surface Science and Nanotechnology (Internet), 13, p.339 - 342, 2015/06
Rare-earth doped ceria exhibits both ionic and electronic conductions, and those ceria with higher ratio of ionic conduction against electronic conduction is used as a solid electrolyte for solid oxide fuel cells. The electron density distributions in crystals are closely related to the electron diffusing pathway which affects the electronic conduction. In this study, we investigated the electron density distribution of doped ceria as a function of the content of Nd O
O -dopant to deduce the ratio of the electronic to ionic conduction. The crystal structure was refined with the space group,
-dopant to deduce the ratio of the electronic to ionic conduction. The crystal structure was refined with the space group, 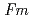 -3
-3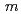 , which is the same as undoped ceria. Ce and Nd ions randomly occupied the 4
, which is the same as undoped ceria. Ce and Nd ions randomly occupied the 4 site and O ion the 8
site and O ion the 8 site. The electron conduction pathway was distributed through the 4
site. The electron conduction pathway was distributed through the 4 -8
-8 and 8
and 8 -8
-8 sites. The relationship between crystal structural change and electron density distribution as a function of the content of Nd
sites. The relationship between crystal structural change and electron density distribution as a function of the content of Nd O
O dopant will be discussed.
dopant will be discussed.
 O
O analyzed by combination of Rietveld/ maximum entropy method
analyzed by combination of Rietveld/ maximum entropy methodIgawa, Naoki; Kodama, Katsuaki; Birumachi, Atsushi; Taguchi, Tomitsugu
e-Journal of Surface Science and Nanotechnology (Internet), 13, p.247 - 252, 2015/05
The nuclear and electron density distributions of LiMn O
O which is one of the primitive cathode materials for secondary Li-ion batteries, were analyzed by applying Rietveld refinement and MEM to neutron and X-ray diffraction data, to estimate the Li diffusing pathway. The crystal structure of LiMn
which is one of the primitive cathode materials for secondary Li-ion batteries, were analyzed by applying Rietveld refinement and MEM to neutron and X-ray diffraction data, to estimate the Li diffusing pathway. The crystal structure of LiMn O
O could be refined with the space group,
could be refined with the space group, 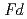 -3
-3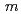 in the temperature range from 240 to 573 K. The structure was transformed to
in the temperature range from 240 to 573 K. The structure was transformed to 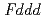 below 240 K. The isotropic thermal displacement parameter of Li was proportional to the temperature excluding 240 to 300 K. According to the MEM analyses it was indicated that the Li ions diffuse through 8
below 240 K. The isotropic thermal displacement parameter of Li was proportional to the temperature excluding 240 to 300 K. According to the MEM analyses it was indicated that the Li ions diffuse through 8 and 16
and 16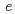 around 300 K.
around 300 K.
 Au; Protective layer formation
Au; Protective layer formationOkada, Michio*; Teraoka, Yuden
no journal, ,
 Al(210) surface at room temperature using supersonic oxygen molecular beam; Real-time photoemission spectroscopic study with synchrotron radiation
Al(210) surface at room temperature using supersonic oxygen molecular beam; Real-time photoemission spectroscopic study with synchrotron radiationSyu, Y.*; Sakurai, Junya*; Teraoka, Yuden; Yoshigoe, Akitaka; Demura, Masahiko*; Hirano, Toshiyuki*
no journal, ,
Zhang, H.; Yamamoto, Shunya; Li, H.; Maekawa, Masaki; Fukaya, Yuki; Kawasuso, Atsuo; Seki, Takeshi*; Saito, Eiji*; Takanashi, Koki*
no journal, ,
Hyodo, Toshio*; Fukaya, Yuki; Mochizuki, Izumi*; Wada, Ken*; Maekawa, Masaki; Shidara, Tetsuo*; Ichimiya, Ayahiko*; Kawasuso, Atsuo
no journal, ,
no abstracts in English
Mochizuki, Izumi*; Fukaya, Yuki; Wada, Ken*; Maekawa, Masaki; Kawasuso, Atsuo; Shidara, Tetsuo*; Hyodo, Toshio*
no journal, ,
no abstracts in English
Wada, Ken*; Maekawa, Masaki; Fukaya, Yuki; Kawasuso, Atsuo; Mochizuki, Izumi*; Shidara, Tetsuo*; Hyodo, Toshio*
no journal, ,
no abstracts in English
 Sr
Sr CoO
CoO
Yoshii, Kenji; Matsumura, Daiju
no journal, ,
Perovskite cobalt oxides have been extensively studied from several viewpoints such as a possible application to solid oxide fueld cells (SOFCs). Recently, Pr Sr
Sr CoO
CoO was proposed to be suitable to SOFCs, on the basis of its high electrical conductivity. In particular, the oxide with x=0.3 has the hightst conductivity, which may be due to the change of electronic structure arising from an increase in oxygen vacancy for x
was proposed to be suitable to SOFCs, on the basis of its high electrical conductivity. In particular, the oxide with x=0.3 has the hightst conductivity, which may be due to the change of electronic structure arising from an increase in oxygen vacancy for x 0.3. In this study, we have measured X-ray absortpion spectra at Pr and Co absorption edges to obtain the information on electronic and local-crystal structures of this system. It was found that the Debye-Waller factor is saturated at around x=0.3 from the measurement at Co K-edge, suggesting a correlation between the atomic vibration and the electrical conductivity. Other data such as the change of valence state against x will be discussed also.
0.3. In this study, we have measured X-ray absortpion spectra at Pr and Co absorption edges to obtain the information on electronic and local-crystal structures of this system. It was found that the Debye-Waller factor is saturated at around x=0.3 from the measurement at Co K-edge, suggesting a correlation between the atomic vibration and the electrical conductivity. Other data such as the change of valence state against x will be discussed also.
Saito, Hiroyuki; Takagi, Shigeyuki*; Aoki, Katsutoshi*; Orimo, Shinichi*
no journal, ,
Teraoka, Yuden; Iwai, Yutaro*; Okada, Ryuta; Yoshigoe, Akitaka
no journal, ,
The decontamination of  Cs is necessary to make volume reduction of radioactive waste. In our research group, chemical bonding states of Cs in clay minerals has been studied by synchrotron X-ray photoemission spectroscopy (SR-XPS). In this presentation, effects of surface charging via an electron flood gun during SR-XPS is discussed. All SR-XPS experiments were conducted at SUREAC2000 of BL23SU in SPring-8. The natural vermiculite produced in Fukushima prefecture, Japan was processed to adsorb Cs by dipping in a non-radioactive CsCl solution. The concentration of Cs was 2.1 wt%. The other Cs compounds, e.g. CsClO
Cs is necessary to make volume reduction of radioactive waste. In our research group, chemical bonding states of Cs in clay minerals has been studied by synchrotron X-ray photoemission spectroscopy (SR-XPS). In this presentation, effects of surface charging via an electron flood gun during SR-XPS is discussed. All SR-XPS experiments were conducted at SUREAC2000 of BL23SU in SPring-8. The natural vermiculite produced in Fukushima prefecture, Japan was processed to adsorb Cs by dipping in a non-radioactive CsCl solution. The concentration of Cs was 2.1 wt%. The other Cs compounds, e.g. CsClO , were also used. The synchrotron radiation of 1486.6 eV, identical with the Al-K
, were also used. The synchrotron radiation of 1486.6 eV, identical with the Al-K line for convenience' sake of comparison with experiments in a laboratory, was used. Sample surface charging was changed by an electron flood gun during SR-XPS. It was revealed that the Auger parameter of Cs of CsClO
line for convenience' sake of comparison with experiments in a laboratory, was used. Sample surface charging was changed by an electron flood gun during SR-XPS. It was revealed that the Auger parameter of Cs of CsClO was closest to that of Cs-contained vermiculite, suggesting Cs atoms adsorbed in the vermiculite may also interact with O atoms contained in the vermiculite. Photoemission spectra of Cs-3d core levels were measured to find four components. The component having the highest binding energy is only shifted by the operation of flood gun, suggesting most ionic chemical bonding. Consequently, the shifted component may be originated from Cs hydrated in water of weathered wide crevice between phyllosilicate layers. The other components are corresponding to interaction with O and Si atoms in narrow phyllosilicate interlayers.
was closest to that of Cs-contained vermiculite, suggesting Cs atoms adsorbed in the vermiculite may also interact with O atoms contained in the vermiculite. Photoemission spectra of Cs-3d core levels were measured to find four components. The component having the highest binding energy is only shifted by the operation of flood gun, suggesting most ionic chemical bonding. Consequently, the shifted component may be originated from Cs hydrated in water of weathered wide crevice between phyllosilicate layers. The other components are corresponding to interaction with O and Si atoms in narrow phyllosilicate interlayers.
 1 surface
1 surfaceYoshigoe, Akitaka; Okada, Ryuta; Teraoka, Yuden; Iwai, Yutaro*; Yamada, Yoichi*; Sasaki, Masahiro*
no journal, ,
Germanium (Ge) has received much attention as promising substitute channel material for future electronic devices. In this conference, we report the nature of oxides of Ge(100)-2x1 surface fabricated with pure oxygen gas (O ) at 300 K. Surface oxide and its evolution were observed by in-situ synchrotron radiation photoelectron spectroscopy (SR-XPS) measurement during oxidation from the very initial stages to the maximum oxide coverage. Incident (O
) at 300 K. Surface oxide and its evolution were observed by in-situ synchrotron radiation photoelectron spectroscopy (SR-XPS) measurement during oxidation from the very initial stages to the maximum oxide coverage. Incident (O ) energy dependence of oxides was investigated using supersonic molecular beam techniques. We found that saturation oxide coverage on Ge(100)is much less than one monolayer. SR-XPS measurements clearly demonstrated that the maximum oxidation number of Ge in saturation region was as small as 2. The population of the Ge2+ and oxygen coverage increased with increasing the incident (O
) energy dependence of oxides was investigated using supersonic molecular beam techniques. We found that saturation oxide coverage on Ge(100)is much less than one monolayer. SR-XPS measurements clearly demonstrated that the maximum oxidation number of Ge in saturation region was as small as 2. The population of the Ge2+ and oxygen coverage increased with increasing the incident (O ) energy. Our findings reveal the significant difference between the nature of surface oxides of Ge and Si.
) energy. Our findings reveal the significant difference between the nature of surface oxides of Ge and Si.
Tang, J.*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Nishimoto, Kiwamu*; Ishizuka, Shinji*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
Tanaka, Kazuma*; Ono, Shinya*; Kodama, Hiraku*; Abe, Sosuke*; Miura, Shu*; Narishige, Takuma*; Yoshigoe, Akitaka; Teraoka, Yuden; Tanaka, Masatoshi*
no journal, ,