Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 - and X-ray measurements by means of Compton suppression technique for safeguards environmental samples
- and X-ray measurements by means of Compton suppression technique for safeguards environmental samplesYasuda, Kenichiro; Mori, Masahito*; Miyamoto, Yutaka; Magara, Masaaki; Sakurai, Satoshi; Usuda, Shigekazu
Journal of Nuclear Science and Technology, 39(Suppl.3), p.576 - 578, 2002/11
no abstracts in English
Sakamoto, Yoshiaki; Ishii, Tomoaki*; Inagawa, Satoshi*; Gunji, Yasuyoshi*; Takebe, Shinichi; Ogawa, Hiromichi; Sasaki, Tomozo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.481 - 484, 2002/11
Sorption behavior of 227Ac and 233Pa onto several kinds of soils has been studied with a sequential extraction technique, for safety assessment of shallow land disposal of uranium bearing waste. After a batch sorption experiment, the sorbed form of 227Ac and 233Pa onto the soils was fractionated into ion exchange form (extraction by KCl+CaCl2), association with Fe+Mn oxides (extraction by NH2OH-HCl and oxalic acid), association with organic materials (extraction by H2O2) and fixation into soil (residual). From the results of the sequential extraction, major part of 227Ac sorbed onto the soils was found in the fraction of the ion exchange form and the fixation into the soils. On the other hand, major part of 233Pa was found in the fraction of the association with Fe+Mn oxides and the fixation into the soils. These results suggest that the sorption behavior of 227Ac and 233Pa is related to the irreversible sorption reaction onto the soils.
Kuwabara, Jun; Tolmachyov, S.; Noguchi, Hiroshi
Journal of Nuclear Science and Technology, 39(Suppl.3), p.556 - 559, 2002/11
no abstracts in English
Omichi, Toshihiko*; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.156 - 159, 2002/11
no abstracts in English
Nakajima, Kunihisa; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.620 - 623, 2002/11
no abstracts in English
Shirai, Osamu; Uozumi, Koichi*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.745 - 748, 2002/11
no abstracts in English
Yamashita, Toshiyuki; Kuramoto, Kenichi; Nakada, Masami; Yamazaki, Satoshi*; Sato, Tsuyoshi*; Matsui, Tsuneo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.585 - 591, 2002/11
no abstracts in English
Tanaka, Tadao; Nagao, Seiya; Sakamoto, Yoshiaki; Ogawa, Hiromichi
Journal of Nuclear Science and Technology, 39(Suppl.3), p.524 - 527, 2002/11
Influence of humic acid on the sorption of Pu onto a coastal sand, which does not sorb humic acid, and an ando soil, which sorbs humic acid very well, was examined with respect to molecular sizes of humic acid. Sorption affinity of Pu for the coastal sand decreased with increasing humic acid concentration. As to the ando soil, the sorption affinity of Pu in the presence of humic acid was larger than that in the absence of humic acid, in low humic acid concentration range. These results suggest that apparent sorption affinity of Pu on the soils is dependent on the complexation ability with humic acid and the sorption affinity of the resulting humic complexes. Concentration profiles of Pu in each size fraction of solution before and after the sorption experiment were obtained by ultrafiltration technique. It was found that the complexation and sorption properties of humic acid are dependent on its molecular size and the important molecular size relating to the complexation and sorption properties tends to shift into smaller size ranges with increasing humic acid concentration.
Kimura, Takaumi; Nagaishi, Ryuji; Ozaki, Takuo; Arisaka, Makoto*; Yoshida, Zenko
Journal of Nuclear Science and Technology, 39(Suppl.3), p.233 - 239, 2002/11
no abstracts in English
Motooka, Takafumi; Kiuchi, Kiyoshi
Journal of Nuclear Science and Technology, 39(Suppl.3), p.367 - 370, 2002/11
A corrosion of a stainless steel in nitric acid solution containing Np has been studied. Using SUS304L stainless steel, corrosion tests in boiling neptunium nitrate solution were conducted under immersion and heat-transfer condition. By the weight loss measurement of stainless steel and the quantitative analysis of metallic ions dissolved in solution, the corrosion rates were obtained. The surface morphology was observed by scanning electron microscopy. The acceleration mechanism was investigated by electrochemistry and spectrophotometry. The corrosion rate was accelerated by addition of neptunium in nitric acid. Preferential intergranular corrosion was observed. The corrosion was enhanced under heat-transfer condition compared to immersion condition. In polarization measurements, the cathodic over-voltage was decreased; the cathodic current was increased by addition of Np. Spectrophotometric measurements showed that Np(V) oxidized to Np(VI) in boiling nitric acid. The corrosion acceleration mechanism in nitric acid solution containing Np suggested the re-oxidation of Np(V).
Hayashi, Hirokazu; Kobayashi, Fumiaki; Ogawa, Toru; Minato, Kazuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.624 - 627, 2002/11
The behavior of the dissolution of uranium nitrides, which is a component of the MA-U mixed nitride fuel, has been investigated. A mixture of uranium nitride, LiCl-KCl and CdCl was contained in a double walled quartz reaction vessel. During the heating, the carrier gas helium was introduced to a gas chromatography apparatus equipped with an automatic gas sampler. A fraction of the gas was sampled and analyzed by a mass analyzer. Most of nitrogen is recovered as N
was contained in a double walled quartz reaction vessel. During the heating, the carrier gas helium was introduced to a gas chromatography apparatus equipped with an automatic gas sampler. A fraction of the gas was sampled and analyzed by a mass analyzer. Most of nitrogen is recovered as N gas from uranium nitrides dissolving in the LiCl-KCl eutectic melt reacted with CdCl
gas from uranium nitrides dissolving in the LiCl-KCl eutectic melt reacted with CdCl above 550
above 550 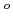 C, which is higher than that for lanthanide nitrides.
C, which is higher than that for lanthanide nitrides.
Okamoto, Yoshihiro; Akabori, Mitsuo; Ito, Akinori; Ogawa, Toru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.638 - 641, 2002/11
We report local structural features of molten UCl with LiCl-KCl eutectic probed by the U L
with LiCl-KCl eutectic probed by the U L -edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U
-edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U -Cl
-Cl distance and the coordination number of Cl
distance and the coordination number of Cl around U
around U ion were obtained by a curve fitting of the 1st shell XAFS function k
ion were obtained by a curve fitting of the 1st shell XAFS function k
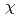 (k). The pair potential in the U
(k). The pair potential in the U -Cl
-Cl correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.
correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.
 M
M O
O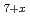 7(M=Pu, Ce) by EMF method
7(M=Pu, Ce) by EMF methodOtobe, Haruyoshi; Nakamura, Akio; Yamashita, Toshiyuki; Ogawa, Toru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.652 - 655, 2002/11
Pyrochlore-type (P-type) zirconia has been widely studied as a disposable form of the high-level radioactive nuclear waste. We have developed the EMF (electromotive force) measurement system using the cubic zirconia sensor, in order to investigate the oxygen potential ( g(O
g(O )) of P-type Zr
)) of P-type Zr Pu(Ce)
Pu(Ce) O
O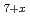 . For P-type Zr
. For P-type Zr Ce
Ce O
O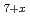 at 1078 K, we have found that
at 1078 K, we have found that  g(O
g(O ) vs. oxygen composition (x) relationship has different behavior between x more than 0.34 and x less than 0.34. This may suggest that the degree and way of the ordering of oxide ions (O
) vs. oxygen composition (x) relationship has different behavior between x more than 0.34 and x less than 0.34. This may suggest that the degree and way of the ordering of oxide ions (O ) and oxygen vacancies (V0) should change around x =0.34. We have also measured the
) and oxygen vacancies (V0) should change around x =0.34. We have also measured the  g(O
g(O ) vs. temperature (T) relationship between 763 and 1078 K at various x, and derived the partial molar enthalpy (h(O
) vs. temperature (T) relationship between 763 and 1078 K at various x, and derived the partial molar enthalpy (h(O )) and entropy (s(O
)) and entropy (s(O )) of oxygen. It was found that h(O
)) of oxygen. It was found that h(O ) and s(O
) and s(O ) of P-type Zr
) of P-type Zr Ce
Ce O
O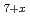 exhibit significantly different behavior from those of fluorite-type CeO
exhibit significantly different behavior from those of fluorite-type CeO . The similar experiments are in progress for P-type Zr
. The similar experiments are in progress for P-type Zr Pu
Pu O
O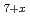 , and the results are discussed in comparison with those of P-type Zr
, and the results are discussed in comparison with those of P-type Zr Ce
Ce O
O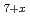 mentioned above.
mentioned above.
 -NaCl and NdCl
-NaCl and NdCl -KCl system by
-KCl system by  ray attenuation
ray attenuationSato, Tadashi; Okamoto, Yoshihiro; Ogawa, Toru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.635 - 637, 2002/11
Densities of molten chlorides mixtures of NdCl -NaCl and NdCl
-NaCl and NdCl -KCl systems have been measured using
-KCl systems have been measured using  -ray attenuation method as a function of temperature. Gamma ray spectra of Ho-166m radiation source were taken before and after passing through the molten chlorides in a quartz cell by a Ge-detector coupled with muluti-channel analyzer. The density was calculated with observed attenuation factor, cross section data of photons for relevant elements and length of the cell. The measured densities of molten NdCl
-ray attenuation method as a function of temperature. Gamma ray spectra of Ho-166m radiation source were taken before and after passing through the molten chlorides in a quartz cell by a Ge-detector coupled with muluti-channel analyzer. The density was calculated with observed attenuation factor, cross section data of photons for relevant elements and length of the cell. The measured densities of molten NdCl -NaCl and NdCl
-NaCl and NdCl -KCl were compared with literature data by dilatometric method.
-KCl were compared with literature data by dilatometric method.

Yamashita, Toshiyuki; Yamazaki, Satoshi*; Sato, Tsuyoshi*; Matsui, Tsuneo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.656 - 659, 2002/11
no abstracts in English
Ito, Akinori; Akabori, Mitsuo; Takano, Masahide; Ogawa, Toru; Numata, Masami; Itonaga, Fumio
Journal of Nuclear Science and Technology, 39(Suppl.3), p.737 - 740, 2002/11
no abstracts in English
 Cm-
Cm- Pu oxide and mononitride
Pu oxide and mononitrideTakano, Masahide; Ito, Akinori; Akabori, Mitsuo; Ogawa, Toru; Numata, Masami; Kizaki, Minoru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.842 - 845, 2002/11
no abstracts in English
 -MO
-MO heterogeneous multi-phase systems (M=Ti, Nb, Si, V, etc.)
heterogeneous multi-phase systems (M=Ti, Nb, Si, V, etc.)Nakamura, Akio; Yoshii, Kenji
Journal of Nuclear Science and Technology, 39(Suppl.3), p.160 - 163, 2002/11
no abstracts in English
Nakamoto, Tadahiro*; Nakada, Masami; Nakamura, Akio
Journal of Nuclear Science and Technology, 39(Suppl.3), p.102 - 105, 2002/11
no abstracts in English
Yasuda, Kenichiro; Sakurai, Satoshi; Gunji, Hideho; Usuda, Shigekazu
Journal of Nuclear Science and Technology, 39(Suppl.3), p.552 - 555, 2002/11
In order to contribute to the strengthened safeguards system based on the Program 93+2 of the IAEA, Japan Atomic Energy Research Institute (JAERI) constructed the CLEAR facility (Clean Laboratory for Environmental Analysis and Research) and is developing analytical technology for ultra trace amounts of nuclear materials in environmental samples. To avoid cross-contamination among the samples and contamination of the clean rooms, radioactive materials in the samples to be introduced into the CLEAR facility will be limited to a certain amount. For this purpose the authors have examined the feasibility of Imaging-plate method, which is a kind of autoradiography and is suitable for determination on distribution of low-level radioactivity in the samples. Preliminary examination with  -ray (K-40), the linearity was obtained in the range of 0.01 - 0.2 Bq. The experiments with
-ray (K-40), the linearity was obtained in the range of 0.01 - 0.2 Bq. The experiments with  -ray (Sm-147) suggested the detection limit of 0.01 Bq, which was equivalent to 2
-ray (Sm-147) suggested the detection limit of 0.01 Bq, which was equivalent to 2  g of natural uranium. At the presentation, the results on actual environmental samples will be reported.
g of natural uranium. At the presentation, the results on actual environmental samples will be reported.