Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tomoyori, Katsuaki; Kusaka, Katsuhiro*; Yamada, Taro*; Tamada, Taro
Journal of Structural and Functional Genomics, 15(3), p.131 - 135, 2014/09
We plan to design a high-resolution biomacromolecule neutron time-of-flight diffractometer, which allows us to collect data from crystals with unit cells above 250  in the Materials and Life Science Experimental Facility at the Japan Proton Accelerator Research Complex. This new diffractometer can be used for a detailed analysis of large proteins such as membrane proteins and supermolecular complex. A quantitative comparison of the intensity and pulse width of a decoupled moderator (DM) against a coupled moderator (CM) considering the pulse width time resolution indicated that the DM satisfies the criteria for our diffractometer rather than the CM. The results suggested that a characteristic feature of the DM, i.e., narrow pulse width with a short tail, is crucial for the separation of Bragg reflections from crystals with large unit cells. On the other hand, it should be noted that the weak signals from the DM are buried under the high-level background caused by the incoherent scattering of hydrogen atoms, especially, in the case of large unit cells. We propose a profile-fitting integration method combined with the energy loss functions and a background subtraction method achieved by employing the Statistics-sensitive Nonlinear Iterative Peak-clipping algorithm.
in the Materials and Life Science Experimental Facility at the Japan Proton Accelerator Research Complex. This new diffractometer can be used for a detailed analysis of large proteins such as membrane proteins and supermolecular complex. A quantitative comparison of the intensity and pulse width of a decoupled moderator (DM) against a coupled moderator (CM) considering the pulse width time resolution indicated that the DM satisfies the criteria for our diffractometer rather than the CM. The results suggested that a characteristic feature of the DM, i.e., narrow pulse width with a short tail, is crucial for the separation of Bragg reflections from crystals with large unit cells. On the other hand, it should be noted that the weak signals from the DM are buried under the high-level background caused by the incoherent scattering of hydrogen atoms, especially, in the case of large unit cells. We propose a profile-fitting integration method combined with the energy loss functions and a background subtraction method achieved by employing the Statistics-sensitive Nonlinear Iterative Peak-clipping algorithm.
Adachi, Motoyasu; Meguro, Mizue*; Maekawa, Shun*; Okazaki, Nobuo*; Beppu, Miho*; Nagasawa, Kazumichi*; Hirano, Ayumi*; Tamada, Taro; Kato, Takashi; Kuroki, Ryota
no journal, ,
The crystal structures of human EPO and human TPO have been determined both as a receptor bound form and a monoclonal antibody bound form, but isolated structures have not been determined. To determine how much structural and functional differences in hematopoietic cell growth factors exist, we are investigating structure and function of EPO and TPO homologues derived from other vertebrates such as amphibian and fish. Recently, we succeeded in preparations of recombinant EPOs from Xenopus laevis (xl-EPO) and Oryzias latipes (ol-EPO) using E. coli expression and Brevibacillus secretion systems, respectively, and both EPOs were successfully crystallized. The structure determinations of xl-EPO and ol-EPO will contribute to further understanding of the structure-function relationships of EPO including the structural change upon receptor binding.
Tomoyori, Katsuaki; Kusaka, Katsuhiro*; Yamada, Taro*; Tamada, Taro
no journal, ,
We performed a time-of-flight (TOF) single crystal neutron diffraction experiment with a diffractometer (the IBARAKI Biological Crystal Diffractometer (iBIX)) installed at a coupled moderator (CM) pulsed neutron source in J-PARC using single crystal silicon, and we determined several candidates for fundamental fitting functions to faithfully reproduce the TOF Bragg reflection profile asymmetries with the longer tail shape. The Vavilov and Landau distributions used to describe the energy loss of charged particles traversing a thin absorber were found to be in excellent agreement with the observed TOF profile. We are planning to design a new TOF single crystal diffractometer installed at a decoupled moderator (DM) pulsed neutron source in J-PARC. The peak profile provides narrow neutron pulses with short tail. In any event, however, it is expected that the Vavilov and Landau functions are very effective and appropriate for use in the TOF distribution of Bragg reflections because there is no fundamental difference in the neutron moderation process between the two kinds of moderator. It is possible to make use of this functions for the integration of Bragg reflection in the case of profile-fitting refinement for protein sample; this functions might be also be applicable to peak separation of the overlapped Bragg reflection in the TOF direction in foreseeable future.
Arai, Shigeki; Yonezawa, Yasushi*; Adachi, Motoyasu; Tamada, Taro; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
no journal, ,
Halophilic proteins have unique structural characteristics: high content of acidic residues creating negatively charged surface, high reversibility of tertiary structure and activity even in high salt concentration, which make it possible to create potent adhesives for metal ions. As part of our structure-function studies for halophilic proteins, we succeeded in crystallization of several halophilic proteins using divalent metal ions as additives. Here, we show successful examples of structural determination of three halophilic proteins: two are alkaline phosphatase from  sp.593 (HaAP) and
sp.593 (HaAP) and  -Lactamase from
-Lactamase from  sp.560 (HaBLA) determined to 2.1
sp.560 (HaBLA) determined to 2.1 and 2.0
and 2.0 resolution, respectively, and the other is nucleoside diphosphate kinase from
resolution, respectively, and the other is nucleoside diphosphate kinase from  sp.593 (HaNDK) determined to 2.3
sp.593 (HaNDK) determined to 2.3 resolution.
resolution.
Arimori, Takao*; Kawamoto, Noriko*; Okazaki, Nobuo*; Nakazawa, Masami*; Miyatake, Kazutaka*; Fukamizo, Tamo*; Ueda, Mitsuhiro*; Tamada, Taro
no journal, ,
Chitin, linear  -1,4-linked polymer of
-1,4-linked polymer of  -acetyl-D-glucosamine (NAG), is the second abundant biopolymer in nature next to cellulose. Hydrolysis of chitin provides useful products,
-acetyl-D-glucosamine (NAG), is the second abundant biopolymer in nature next to cellulose. Hydrolysis of chitin provides useful products,  -acetyl-chitooligosaccharides [(NAG)
-acetyl-chitooligosaccharides [(NAG) ] and chitooligosaccharides, which have a variety of biological functions including antibacterial activity and antitumor activity. We have previously cloned a novel chitinase gene from a moderate thermophilic strain
] and chitooligosaccharides, which have a variety of biological functions including antibacterial activity and antitumor activity. We have previously cloned a novel chitinase gene from a moderate thermophilic strain 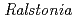 sp. A-471 (Ra-ChiC). Ra-ChiC comprises a signal peptide, a chitin-binding domain, an interdomain linker, and a catalytic domain. The catalytic domain shares amino acid sequence homology with goose type (G-type) lysozymes and, unlike other chitinases, Ra-ChiC belongs to glycohydrolase (GH) family 23. However, Ra-ChiC does not exhibit lysozyme activity, but only chitinase activity. In this study, we aim to reveal how Ra-ChiC catalyzes the hydrolysis of chitin and why Ra-ChiC exhibits chitinase activity instead of lysozyme activity. We determined the crystal structures of the catalytic domain of Ra-ChiC (Ra-ChiC
sp. A-471 (Ra-ChiC). Ra-ChiC comprises a signal peptide, a chitin-binding domain, an interdomain linker, and a catalytic domain. The catalytic domain shares amino acid sequence homology with goose type (G-type) lysozymes and, unlike other chitinases, Ra-ChiC belongs to glycohydrolase (GH) family 23. However, Ra-ChiC does not exhibit lysozyme activity, but only chitinase activity. In this study, we aim to reveal how Ra-ChiC catalyzes the hydrolysis of chitin and why Ra-ChiC exhibits chitinase activity instead of lysozyme activity. We determined the crystal structures of the catalytic domain of Ra-ChiC (Ra-ChiC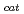 ), Ra-ChiC
), Ra-ChiC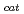 complexed with (NAG)
complexed with (NAG) , E141Q mutant of Ra-ChiC
, E141Q mutant of Ra-ChiC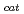 complexed with (NAG)
complexed with (NAG) , E162Q mutant of Ra-ChiC
, E162Q mutant of Ra-ChiC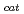 , and E162Q mutant of Ra-ChiC
, and E162Q mutant of Ra-ChiC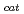 complexed with (NAG)
complexed with (NAG) . These structures provided us structural basis of substrate recognition mechanism and revealed that Ra-ChiC has a unique substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. In addition, we also carried out a mutation analysis of acidic amino acid residues located at the active site. As a result, we found that not only a highly conserved Glu141 but also Asp226 located at the roof of the tunnel have quite important roles in catalysis.
. These structures provided us structural basis of substrate recognition mechanism and revealed that Ra-ChiC has a unique substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. In addition, we also carried out a mutation analysis of acidic amino acid residues located at the active site. As a result, we found that not only a highly conserved Glu141 but also Asp226 located at the roof of the tunnel have quite important roles in catalysis.
Shibazaki, Chie; Adachi, Motoyasu; Kuroki, Ryota
no journal, ,
For elucidation of the molecular recognition and catalytic mechanism of biomolecules, not only the detailed molecular structure, but also the hydration structure of the catalytic site and the exact position of hydrogen atoms are important. Collaborative use of both neutron and X-ray crystallography is a powerful methodology to obtain this information. In such experiments, high quality single crystals with large volume enable us to obtain high resolution X-ray diffraction by reducing the effect of X-ray damage, and improve neutron diffraction data by increasing diffraction intensity. Two different approaches have been attempted to obtain large crystals. One is the periodic addition of protein solution to the mother liquor, and the other is to increase the volume of mother liquor. We report the result of increase of the mother liquor volume after focusing on additives and pH to control the nucleation of protein crystals using several proteins.
 reductase by X-ray crystallography
reductase by X-ray crystallographyTamada, Taro; Yamada, Mitsugu*; Takeda, Kazuki*; Matsumoto, Fumiko*; Kimura, Shigenobu*; Kuroki, Ryota; Miki, Kunio*
no journal, ,
NADH-cytochrome  reductase (b5R) is a flavoprotein consisting of NADH- and FAD- domains, and catalyzes the electron transfer from two electron carriers of NADH to one electron carrier of cytochrome
reductase (b5R) is a flavoprotein consisting of NADH- and FAD- domains, and catalyzes the electron transfer from two electron carriers of NADH to one electron carrier of cytochrome  (Cb5). The structure of the fully reduced form of porcine liver b5R was determined using a crystal grown under anaerobic condition. In the reduced b5R structure, which was determined at 1.68
(Cb5). The structure of the fully reduced form of porcine liver b5R was determined using a crystal grown under anaerobic condition. In the reduced b5R structure, which was determined at 1.68  resolution, the relative location of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent accessible surface area of FAD, and created a new hydrogen bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat, which is similar to that in the oxidized form, and is stacked with the nicotinamide ring of NAD
resolution, the relative location of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent accessible surface area of FAD, and created a new hydrogen bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat, which is similar to that in the oxidized form, and is stacked with the nicotinamide ring of NAD . Both of reduced and oxidized b5R structures could explain how prevents the backflow and accelerates the transfer of electrons to one-electron acceptors, such as Cb5, in the catalytic cycle. Furthermore, the crystallographic analysis by the cryo-trapping method, which controls the exposure time of the fully reduced crystals against the air, suggested that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
. Both of reduced and oxidized b5R structures could explain how prevents the backflow and accelerates the transfer of electrons to one-electron acceptors, such as Cb5, in the catalytic cycle. Furthermore, the crystallographic analysis by the cryo-trapping method, which controls the exposure time of the fully reduced crystals against the air, suggested that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.